A Mini-Review 5-Amino-N-Substituted Pyrazoles as Building Blocks for Bioactive Molecules ()
1. Introduction
Synthesis of heterocyclic compounds is of critical importance due to their fundamental role in pharmaceutical applications. Pyrazoles and their derivatives are an important category of heterocyclic compounds that has gained significant attention recently among pharmacological, medicinal, and biological chemists. Most pyrazole derivatives exhibit significant anticancer properties [1] [2] [3] [4].
Functionally, pyrazoles are used as the starting materials or building blocks of other heterocyclic nitrogen systems such as imidazole pyrazoles [5] and pyrazolyl-1,2,4-triazines [6]. Some amino-pyrazolones such as 4-aminoantipyrine are used, for instance, as agrochemicals [7] in dyestuffs [8] as antipyretics [9], and as α-aminophosphonic acids [10].
This review reports several routes of synthesis of various 5-amino-N-substituted pyrazole derivatives and highlights their behavior toward electrophilic and nucleophilic reagents, to which their highly active biological and pharmaceutical characteristics are due.
2. Synthesis of 5-Amino-N-Substituted Pyrazoles
Treatment of 2-hydrazino-1-phenylethanol 1 with ethyl (ethoxymethylene) cyanoacetate 2 in dry toluene at 80˚C for 8 h produces ethyl 5-amino-1- (2-hydroxy-2-phenylethyl)-1H-pyrazole-4-carboxylate 3 (Scheme 1) [11].
The condensation of hydrazine acetaldehyde diethylacetate 4 with ethyl (ethoxymethylene) cyanoacetate 2 produces 5-amino-1-(2,2-diethoxyethyl)-1N- pyrazole-4-carboxylate 5 (Scheme 2) [12].
Treatment of compound 2 with (ethoxy methylene) malononitrile 6 yields 5-amino-1-(2,2-diethoxyethyl)-1H-pyrazole-4-carbonitrile 7 (Scheme 3) [13].
Treatment of hydrazine derivatives 2 with ethyl cyano pyruvate 8 in a biphasic water/chloroform solvent in the presence of sulfuric acid results in ethyl 5-amino-1-(2,2-diethoxyethyl-1H-pyrazole-3-carboxylate 9 (Scheme 4) [14].
3-Amino-5-phenylpyrazole 11 is obtained from reaction of benzoic acid hydrazide with chloroacetonitrile (Scheme 5) [15].
3. Behaviour of 5-Amino-1-Substituted Pyrazoles towards Electrophilic and Nucleophilic Reagents
Treatment of most 5-amino-1-substituted pyrazoles with electrophilic or nucleophilic reagents led to direct formation of imidazole pyrazoles, which are candidates for considerably highly bioactive systems.
Thus, formylation of amino-1-(2-hydroxyethyl)pyrazole 12, when treated with Ac2O–HCOOH, followed by cyclisation with NaH, produced 1-formyl-2,3- dihydro-1H-imidazo[1,2-b]pyrazole 13 (Scheme 6) [16].
The aza-Wittig reaction of 5-amino-3-phenyl-1H-pyrazole 11 via P(Ph)3/C2Cl6/ Et3N at reflux with benzene produces 5-(triphenyl phosphoranylideneamino)-3- phenylpyrazole 14, which upon treatment with 𝛲-chloroketone yields imidazo[1,2-b]pyrazoles 15 (Scheme 7) [17].
Dehydration of aminopyrazole 3 by treatment with concentrated H2SO4 at room temperature produces ethyl 2-phenyl-2,3-dihydro-1H-imidazo[1,2-b]pyrazole- 6-carboxylate 16 (Scheme 8) [11].
5-Amino-3-phenyl-1H-pyrazole 11 when reacted with hydroximoyl chloride 17 in ethyl alcohol at room temperature yields 3-nitroso-2-aryl-6-phenyl-1H- imidazolo [1,2-b]pyrazoles 18 (Scheme 9) [13].
Acylation of 5-amino-3-substituted-1H-pyrazole 19 using chloroacetyl chloride in basic medium produces 3H-imidazo[1,2-b]pyrazo-2-ol 20 (Scheme 10) [18].
Heterocyclisation reaction of compound 11 by treatment with oxaldiimidoyl dichlorides 21 in THF-TEA yields 3H-imidazo[1,2-b]pyrazoles 22 (Scheme 11) [19].
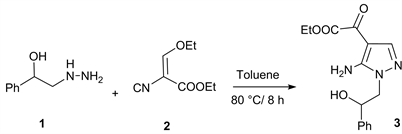
Scheme 1. Using 2-hydrazino-1-phenylethanol with (Ethoxymethylene) cyanoacetate, to produce 5-amino-N-aryl substituted pyrazoles [11].
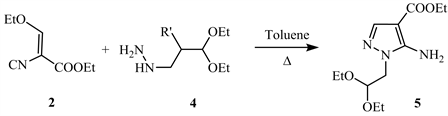
Scheme 2. Condensation of α,β-unsaturated cyanoacetate 2 with hydrazine derivative 4produced 5-amino-N-substituted-4-carboxylate pyrazole 5 [12].
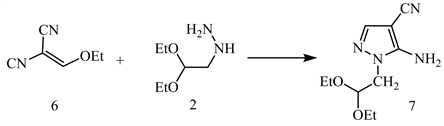
Scheme 3. Compound 2 treated with (ethoxy methylene)malononitrile to afford compound 7 [13].
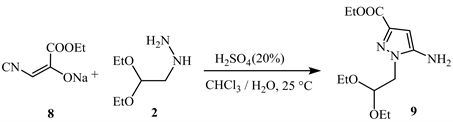
Scheme 4. Using compound 2 derivatives and ethyl cyano pyruvate 8 [14].
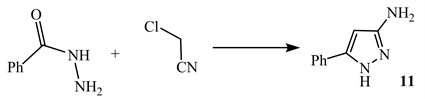
Scheme 5. Using chloroacetonitrile and benzoic acid hydrazide afforded5-amino pyrazole 11 [15].
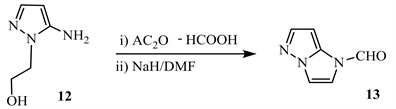
Scheme 6. Formylation of amino group of compound 12 [16].
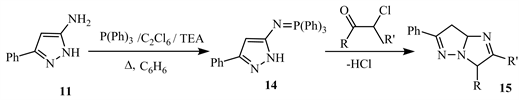
Scheme 7. Carrying the aza-Wittig reaction of 5-amino-3-phenyl-1H-pyrazole 11 [17].
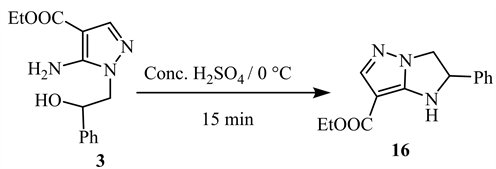
Scheme 8. Under dehydration of aminopyrazole 3 [11].
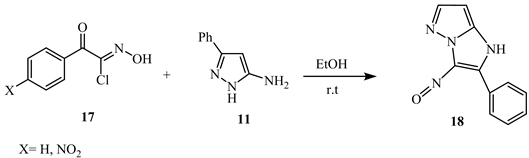
Scheme 9. 1,3-Cycloaddition reaction with α-ketohydroximoyl chloride 17 [13].
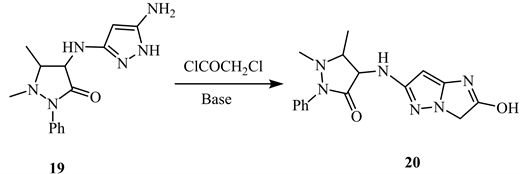
Scheme 10. Acylation of 5-amino-3-substituted-1H-pyrazole 19 [18].
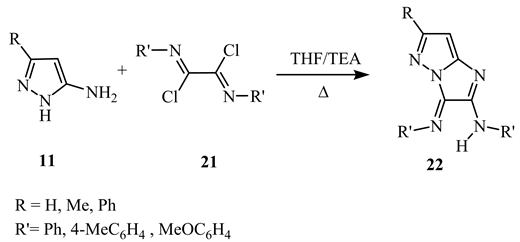
Scheme 11. Heterocyclisation reaction of compound 11 [19].
Cycloaddition reactions of 3,5-diamino-1H-pyrazole-4-carbonitrile 23 with 3,4-dimethoxy benzonitrile 24 in the presence of 2,4-dihydro-2-oxo-1H-benzo [d][1,3]oxazine-7-carboaldehyde 25 results in the substituted imidazo[1,2-b] pyrazole-4-carbonitrile 26 (Scheme 12) [20].
Similarly, the cyclocondensation between aromatic aldehyde, 5-aminopyrazole 11 and isocyanide in acetonitrile with 4-toluene sulfonic acid as a catalyst at room temperature yields N-alkyl-2-aryl-5H-imidazolo[1,2-b]pyrazole-3-amine 27 (Scheme 13) [21].
Heterocyclisation of 5-amino-4-ethoxy-carbonyl amino-pyrazole 28 when fused at 200˚C for 24 h, yielded imidazole[4,5-c]pyrazole 30 gave the imidazole[4,5-c] pyrazole-5-thione 31 (Scheme 14) [22].
When compound 11 is treated with imidoyl chloride 32 in dry dioxane at room temperature in TEA, the substituted amine 33 is produced, which upon treatment with I2/KOH/DMF, yields 1-substituted-5-imino-4-iodo-pyrazole 34. Addition of CuI to 34 yields imidazo[1,2-c]pyrazole 35 (Scheme 15) [23].
Acylation of 5-amino-3-methyl-pyrazole 36 with acetic anhydride or benzoyl chloride yields 5-acyl amino pyrazoles 37. Reduction of compound 37 with LiAlH4 produces the corresponding 5-alkyl amino pyrazoles 38. Nitrosation of 38 via amyl nitrile in HCl yields 5-alkylamino-4-nitrosopyrazole 39, which upon cyclocondensation by reflux with dry pyridine yields imidazo[4,5-c]pyrazole 40 (Scheme 16) [24].
Interesting multi-component reactions (MCRs) were promoted via Knoevenagel condensation; 5-aminopyrazoles 41 have been utilized as 1,3-dinucleophilic starting material with either electron-withdrawing or electron-donating groups and various 1,3-dicarbonyl compounds in a one pot condensation in catalyzed water using ceric ammonium nitrate (CAN) produced a series of spiro[indoline- 3,4’-pyrazolo[3,4-b]quinolone]dione derivatives 44 with good yields under mild conditions and eco-friendly general procedures as shown in Scheme 17 [25].
Around 24 examples of spiro-heterocyclic compounds were synthesised following this simple one-pot protocol, as illustrated in Scheme 18.
In a related synthesis of spiro-heterocyclic compounds which seems to be an improvement over the earlier procedure and is more region-selective, MCRs
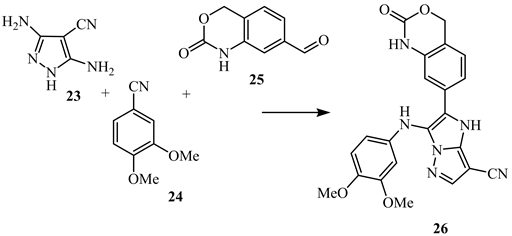
Scheme 12. Cycloaddition reactions of compound 23 [20].
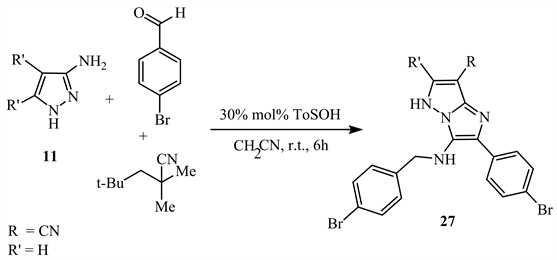
Scheme 13. Cyclocondensation between compound 11 and an isocyanide [21].
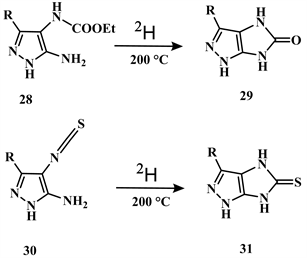
Scheme 14. Heterocyclisation of compounds 28 and 30 yielded imidazole[4,5-c]pyrazole derivatives [22].
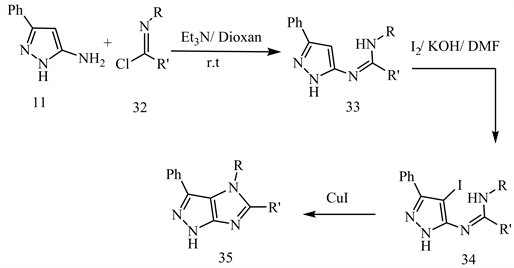
Scheme 15. Nucleophilic substitution of the halogen atom in the pyrazole nucleus 34 to synthesize Imidazo[4,5-c]pyrazoles 35 [23].
were accomplished using 5-amino-3-methyl-1-phenylpyrazole 49, β-diketones 50, and isatin 51 in a similar reaction catalyzed by water using p-TSA, which produced a series of derivatives of pyrazolopyridine-spiroindolinone (Scheme 19) [26].
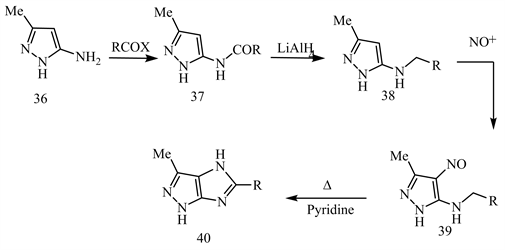
Scheme 16. Acylation of 5-amino-3-methyl-pyrazole 36 [24].

Scheme 17. Knoevenagel condensation of 5-aminopyrazoles 41 [25].
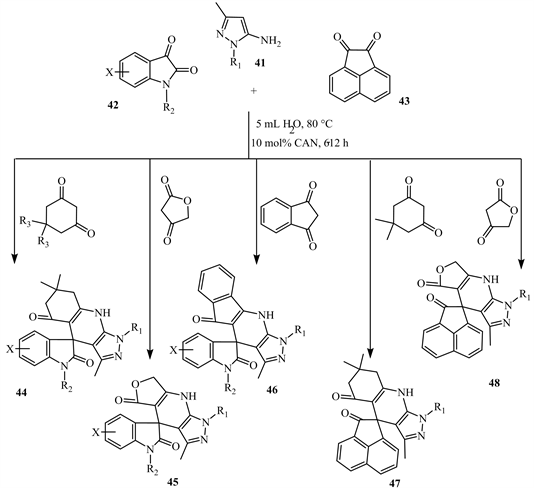
Scheme 18. Spiro-heterocyclic compounds were synthesized using compound 41 [25].
![]()
Scheme 19. A series of pyrazolopyridine-spiroindolinone derivatives were obtained using MCRs [26].
The structure-activity relationship (SAR) of amino-pyrazoles 54 as shown in Figure 1 has been approved as showing effective inhibition of p38a MAP kinase with very good cellular potency, which makes it an effective drug candidate for control of inflammatory disorders [27].
The use of 5-amino-pyrazole moiety led to the synthesis of designed scaffolds which produced potent selective p38α inhibiting drugs. The traditional procedure was used to prepare 5-amino-pyrazoles through condensation reaction of hydrazine 55 along with ethyl (ethoxymethylene) cyanoacetate 2 in ethyl alcohol in the presence of a base such as trimethylamine (Scheme 20). Hydrolysis to carboxylic acid before coupling with aniline using either HATU coupling or converting the acid carboxylic group to acid chloride and thereafter coupling it with aniline derivative 58 afforded good yields of the target compound 59 [27].
Successful one-pot synthesis of structural analogues to purines was achieved via a diazotization reaction which was carried out on 5-amino-1H-pyrazole-4- carbonitriles for the preparation of pyrazolo[3,4-d][1,2,3]triazin-4-ones 60 with moderately good to very good yields (Scheme 21). The reaction proceeded through diazoic acid or diazocarboxamide as intermediates. Various prepared pyrazolo[3,4-d][1,2,3]triazines have been shown to possess useful heterocyclic compounds with broad biological activities, for instance, anticonvulsant, antifungal, antiviral, and anticancer activities and antimicrobial effects [28] [29].
In addition, 5-amino-pyrazo derivatives were used in the synthesis of pyrazolo [3,4-d]pyrimidines in 244 examples [30]. Condensation of 5-heterylaminopyrazoles 64 with some carbonyl compounds in the presence of AcOH or Me3SiCl successfully produced pyrazolo[3,4-d]dihydropyrimidines 65 in good yields (Scheme 22). The best proposed mechanism explaining that reaction includes two steps: first, the reaction of 5-aminopyrazoles 62 with 2-halogenazines or azoles 63 in NaH/THF conditions, followed by ring closure with carbonyl compounds in Me3SiCl or AcOH medium.
4. Importance and Applications
Since the 1960s, the pyrazole moiety has been known to exhibit anticancer activity;
![]()
Figure 1. X-ray showed the expected pyrazolo-pyrimidine 53 and amino-pyrazoles 54.
![]()
Scheme 20. HATU coupling of compound 57 with aniline derivative 58 afforded good yields of the amino-pyrazoles derivatives targeted 59 [27].
![]()
Scheme 21. One-pot diazotization reaction carried out on 5-amino-1H-pyrazole-4- carbonitriles 60 to synthesize pyrazolo[3,4-d][1,2,3]triazin-4-ones 61 [28].
![]()
Scheme 22. The arylation of 1-substituted-5-aminopyrazoles with electrophilic hetarylhalides, then condensation of 5-hetarylaminopyrazoles with carbonyl compounds led to the synthesis of 244 examples [30].
it has also been shown to be an antitumor agent in Phase I studies. However, it was confirmed to be too toxic for human use, even in doses as low as 0.15 mmol/kg/day [31]. Researchers are keen to harness the powerful anticancer activity of pyrazoles without the toxic side effects, and synthesis of a variety of pyrazole derivatives are key to this pursuit [32]. Various derivatives have been tested, among them 1-thiocarbamoylpyrazole, 1-carboxamidopyrazole, which failed to show non-toxicity to humans, although it showed a remarkable anticancer clinical effect [33]. Compounds such as 5-amino-1-cyanoacetyl-3- (p-chlorophenyl)-pyrazole have been tested toward three human tumour cell lines: breast adenocarcinoma (MCF-7), non-small cell lung cancer (NCI-H460), and CNS cancer (SF-268), and it has shown potent inhibition in growth of human tumour cell lines as well [34].
Pyrazole nucleosides and condensed pyrazole nucleosides possess various biological activities which make them powerful drug candidates, and these have been tested toward various diseases caused by DNA viruses [35].
Photographic material can be used to improve the absorption of imidazo[1,2-b] pyrazoles [36] [37] [38] [39]. Also, some imidazo-pyrazoles have antiviral activity against herpes simplex virus type 1 infected mammalian cells [40] and show antimicrobial activity [41] [42]. 2,3-Dihydro-1H-imidazolo[1,2-b]pyrazoles exhibit in vivo effects on the proliferation of mouse leukemic cells [43] [44].
3-Amino-6-(B-D-ribofuranosyl)imidazo[4,5-c]pyrazole, used as a 5:5 fused analogue of adenosine was evaluated against two herpes viruses, herpes simplex virus 1 (HSV-1) and human cytomega covirus (HCMV) [45].
5. Conclusion
In summary, various routes exist for synthesis of 5-amino-N-substituted pyrazoles, which exhibit important chemical behavior towards electrophilic and nucleophilic reagents. Their tendency toward heterocyclisation reactions leads to the direct formation of imidazopyrazoles, which exhibit a variety of significant biological activities. The usefulness of 5-amino-N-substituted pyrazole molecules in the effort to obtain new heterocyclic compounds that have more accessible biocidal effects and enhancements is governed by substituent position and type.