The Properties of Elasticity, Thermology, and Anisotropy in Pd-Based Alloys ()
1. Introduction
Heusler alloys are composed of a series of intermetallics. In recent years, plenty of magnetic properties that Heusler alloys presented and their applications in spintronic devices had aroused wide concern [1] [2] [3] . The properties of Heusler alloys were well diversified, such as non-ferromagnetic elements could exhibit ferromagnetism after highly ordered [4] [5] , 100% spin polarization were presented in materials which were called half-metallic ferromagnets (HMF) [6] [7] , only a minority of Heusler alloys containing rare earth had been reported to be superconductors [8] [9] [10] , etc. These above features elucidated their potential for future applications in different fields.
As early as 1903, Cu2MnAl became the prototype of Heusler alloys, since F. Heusler [11] firstly reported Cu2MnAl and high magnetic ordered alloy of Cu2MnAl series. In 1969, P. Webster [12] discussed magnetic and structure properties of Heusler alloys systematically. Liu et al. [13] discovered another highly ordered Heusler alloy, which named Hg2CuTi. Up to now, more than one hundred kinds of Heusler alloys were found both in theory and experiments, such as Mn-based alloys [14] [15] [16] , Co-based alloys [17] [18] , Cu-based alloys [19] [20] [21] , Ni-based alloys [22] [23] . But the reports about Pd group Heusler alloys were relatively less and the majority of them were related to experiments. Kierstead et al. [24] , Aoki et al. [25] [26] and Stanley et al. [8] studied the Heusler compound Pd2SnYb and Pd2SnEr, whose superconductivity and antiferromagnetism were concomitant. Novel properties of thermodynamics and transmission were shown in Pd2SnYb obviously. And the superconductivity presented at Tc = 2.3 K, along with a synchronous phase of antiferromagnetism and superconductivity yielding at TN = 220 mK. The testing of elastic and inelastic neutron scattering for Pd2SnEr was carried out, which proved that Pd2SnEr turned into superconductor at Tc = 1.17 K. Only when temperature conditions met T > Tc, the antiferromagnetic correlations would occur. The maximum critical temperature was found in Pd2YSn, which was revealed as the Heusler alloy [27] .
However, in the aspect of theoretical calculation, there is no systematic research on elasticity, thermal properties and anisotropy of Pd-based alloys PdSnYb, PdSn2Yb and Pd2SnYb so far. In this work, we provide the overall calculation and analysis of these properties. Especially, once the thermal conductivity is smaller, the heat-shielding performance will be better. The computed minimum thermal conductivities of Pd2SnYb, PdSnYb and PdSn2Yb are all less than 0.5 W・m−1・K−1. This minimum thermal conductivity is small enough to be applied to thermal barrier coatings and many other fields. Hence, the thorough discussion carried on the three materials is essential, which inspires our passion on studying these materials. And it makes great sense to explore the microstructure and properties of Pd-based alloys.
2. Calculation Model and Parameters
2.1. Model Details
Pd-based system used in this work includes three alloys: Pd2SnYb, PdSnYb and PdSn2Yb. The symmetry group and international table number of Pd2SnYb are 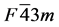 and 216. PdSnYb and PdSn2Yb are orthorhombic system. Their space groups are PNMA (No. 62) and CMCM (No. 63). In Pd2SnYb, Pd possesses the 8c site (0.25, 0.25, 0.25), Sn perches the 4a site (0, 0, 0), Yb possesses the 4b site (0.5, 0.5, 0.5). In PdSnYb, atoms of Pd, Sn, and Yb respectively possess the 4c site (0.28675, 0.25, 0.3988), (0.17329, 0.25, 0.07563) and (0.0176, 0.25, 0.69161). In PdSn2Yb, Pd and Yb perch the 4c site (0, 0.70228, 0.25), (0, 0.42899, 0.25), Sn possesses the 8f site (0, 0.14024, 0.04483). In order to obtain reliable structural optimization results, the lattice constants we employed are all from experiments.
and 216. PdSnYb and PdSn2Yb are orthorhombic system. Their space groups are PNMA (No. 62) and CMCM (No. 63). In Pd2SnYb, Pd possesses the 8c site (0.25, 0.25, 0.25), Sn perches the 4a site (0, 0, 0), Yb possesses the 4b site (0.5, 0.5, 0.5). In PdSnYb, atoms of Pd, Sn, and Yb respectively possess the 4c site (0.28675, 0.25, 0.3988), (0.17329, 0.25, 0.07563) and (0.0176, 0.25, 0.69161). In PdSn2Yb, Pd and Yb perch the 4c site (0, 0.70228, 0.25), (0, 0.42899, 0.25), Sn possesses the 8f site (0, 0.14024, 0.04483). In order to obtain reliable structural optimization results, the lattice constants we employed are all from experiments.
2.2. Parameters Setting
CASTEP code [28] was used for this work, which grounded on the density functional theory [29] . The exchange correlation functional employed the PBE method in the generalized gradient approximation (GGA) [30] . Ultra soft pseudo potential (USPP) [31] was chosen for interaction potential between ionic potential and valence electrons. The atom orbits Pd 4d10, Sn 5s25p2, and Yb 4f145s25p66s2 were considered as valence electrons in the calculation of pseudo potential. The cut-off energy of 450 eV was set for plane waves in the wave-vector K space. For Brillouin regions k-point sampling, the Monkhors-Pack mesh was set as 4 × 4 × 4 [32] . The lattice parameters of Pd2SnYb, PdSnYb and PdSn2Yb were optimized successively by using the BFGS scheme [33] [34] [35] [36] . On this basis, the magnetic, alloy corrosion resistance, elastic, thermal conductivity and anisotropy are being computed.
3. Calculation Results and Discussions
3.1. Magnetic Property
The equilibrium lattice constants of Pd2SnYb, PdSnYb, and PdSn2Yb are obtained by geometry optimization with spin polarization. The paramagnetic (NM), ferromagnetic (FM) and anti-ferromagnetic (AFM) coupling between Yb atoms are taken into account in the calculations. Atomic initial magnetic order affects the convergence of ground state. Therefore, the different magnetic orders of Yb atoms are considered to ensure the convergence of ground state. In the condition of different magnetic orders, the curves of the relative energy are drawn out in Figure 1, whose minimum energy is set up to be the ground state (0 eV).
As the Figure 1 shown, the energy of AFM-2 (each layer of Yb atoms spin in the opposite manner along the crystal orientation [001]) in Pd2SnYb is higher than other magnetic orders. And this proves spin polarization displaying in Pd2SnYb. However, the energy of NM in PdSnYb and PdSn2Yb are the highest. It demonstrates the ground state of these three materials, which is in accordance
![]()
Figure 1. The energy curve of NM, FM and AFM in Pd2SnYb, PdSnYb and PdSn2Yb.
with the experiments [37] [38] . The calculated magnetic moment value of each Yb is 2.4 μB for the alloy composition Pd2SnYb. The total magnetic moment of per formula unit for Pd2SnYb and atoms Yb, Pd, Sn are all 0 μB. As we can see, Pd2SnYb is barely the magnetic one among the three Pd-based alloys. According to Mulliken’s bond population and length shown in Table 1, Pd2SnYb contains the bond type Pd-Yb, which the other two alloys don’t. And the lack of Pd-Yb bonding maybe indicates the reason for appearing a transition from non-mag- netism (Pd2SnYb, Pd2SnYb) to anti-ferromagnetism (Pd2SnYb). The bond population means the distribution of overlapping electron charge between two atoms. It is usually used to evaluate the ionicity or covalency of a bond. Compared with the positive values of Pd-Sn, the negative of Pd-Yb displays its iconicity, which is also connected with the magnetism in Pd2SnYb.
3.2. Structural Parameters
Based on the calculation of magnetic ground state in 3.1 Magnetic properties, lattice constant, volume, density, total energy, cohesive energy, formation enthalpy and partial experiment values of Pd2SnYb, PdSnYb and PdSn2Yb are listed in Table 2. As we all know, GGA calculation usually overestimates lattice constants. On the contrary, the elastic constants are underestimated. Therefore, lattice parameters calculated by GGA are slightly larger. While the error is negligible, and the computed results still agree well with the experiment data.
For further details of the bonding properties in these alloys, the cohesive energy and formation enthalpy per atom of Pd, Sn and Yb atoms are defined
![]()
Table 1. Mulliken’s bond population and length (Ǻ) of the Pd-based alloys.
![]()
Table 2. The calculated lattice constants a, b, c (Å), volume V (Å3), density ρ (g・cm−3), Etot (eV/atom), ΔH (eV), ΔEcoh (eV) and partial experiment values of Pd-Sn-Yb.
as the calculated Equation (1) and (2) [42] [43] .
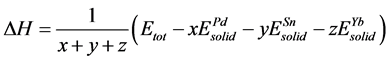 (1)
(1)
 (2)
(2)
Here, ΔH and ΔEcoh respectively represent the formation enthalpy and cohesive energy of Pd-based compounds. Etot stands for the energy of a unit cell.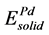 ,
,  ,
, 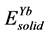 are the energy of each Pd, Sn and Yb atom in the bulk state, and
are the energy of each Pd, Sn and Yb atom in the bulk state, and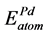 ,
, 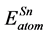 ,
, 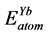 show the total energy of insular Pd, Sn, Yb atom, respectively. x, y and z are the number of Pd, Sn, and Yb atom in unit cell.
show the total energy of insular Pd, Sn, Yb atom, respectively. x, y and z are the number of Pd, Sn, and Yb atom in unit cell.
It is clear that the calculated formation enthalpy and the cohesive energy given in Table 2 are negative: 0 > Pd2SnYb > PdSnYb > PdSn2Yb. The results show that Pd2SnYb, PdSnYb and PdSn2Yb are all thermally stable. Among them, PdSn2Yb is the easiest to synthesis and the most stable alloy. And Pd2SnYb, which has the poorest stability and reacts easily with Cl− or H+ resulting in corrosion, is just on the contrary.
3.3. Fermi Energy
Fermi level (Ef) also can be known as the Fermi energy. If the electrons accumulation in semiconductor is regarded as a thermodynamic system, the statistic theory has been proved that Fermi energy is the electronic chemical potential of this system.
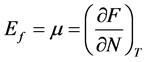 (3)
(3)
in which μ is the chemical potential, F is the free energy, N represents the total number of electrons, T is temperature.
The corrosion behavior on alloys is complicated. In the light of the electron theory, each fermion obeys Fermi-Dirac statistics. According to Pauli exclusion- principle, the minimal energy principle, and Hund rule, fermion occupies the quantum state respectively. On behalf of the top level of electron filling, Fermi energy loses electron in the first. And the higher Fermi level reaches, the easier outermost shells are to lose.
Fermi energy of Pd2SnYb, PdSnYb, and PdSn2Yb are shown in Figure 2. Due to the different types and structures of the system, Fermi level is different in the ground state. Corrosion potential is bound on with the Fermi level. So the higher Fermi level reaches, the smaller corrosion potential will be. As shown in Figure 2, the Fermi energy (Ef) values of these compounds with Ef (PdSnYb) > Ef (Pd2SnYb) > Ef (PdSn2Yb) indicate that PdSnYb is most likely to lose electrons, while PdSn2Yb is difficult. Their corrosion potential and complexity of corroding are in the order of PdSn2Yb > Pd2SnYb > PdSnYb.
3.4. Elastic Property
The reaction to external stress in the elastic limit of crystal lattice can be charac-
![]()
Figure 2. Fermi energy of Pd2SnYb, PdSnYb and PdSn2Yb.
terized by elastic constants. It’s of important significant on the stability and stiffness of materials. Table 3 lists the elastic constants of these three alloys. The elastic constants of cubic and orthorhombic system need to satisfy the generalized stability criteria which can be expressed as [44] :
For cubic phase (Pd2SnYb):
 (4)
(4)
For orthorhombic phase (PdSnYb and PdSn2Yb):

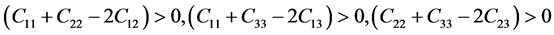 (5)
(5)
As mentioned in Table 3, the mentioned Pd-based alloys are stable in mechanics, due to the elastic constants satisfy the corresponding stability criterions.
It’s well known that the elastic constants C11 and C33 are depicted as the ability to resist linear compression along x and z-axis [45] . The present C11 is equal to C33 in Pd2SnYb, indicating that the compression of x and z-axis is isotropy. The largest C11 of PdSnYb implies that it is the most incompressible material along x-axes obviously. For PdSn2Yb, the value of C33 is slightly higher than the C11, which indicates that the z-axis is less compressible than x-axis. The calculated elastic constants of Pd2SnYb follow the order: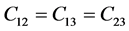
![]() , suggesting the anisotropies of shear moduli for Pd-based intermetallics are relatively weak. The PdSnYb is in the order of
, suggesting the anisotropies of shear moduli for Pd-based intermetallics are relatively weak. The PdSnYb is in the order of![]() . Thus, the bonding strength of adjacent atoms are the highest in (1 0 0) plane. The present C22 is higher than the C11 and C22 for PdSn2Yb. Therefore, the bonding strength of (0 1 0) plane is higher than (1 0 0) and (0 0 1) planes. In conclusion, all the three compounds have the highest binding strength in (1 0 0) plane.
. Thus, the bonding strength of adjacent atoms are the highest in (1 0 0) plane. The present C22 is higher than the C11 and C22 for PdSn2Yb. Therefore, the bonding strength of (0 1 0) plane is higher than (1 0 0) and (0 0 1) planes. In conclusion, all the three compounds have the highest binding strength in (1 0 0) plane.
Additionally, C44, which measures the ability to resist monoclinic shear strain
![]()
Table 3. Elastic constants (GPa) of the three Pd-Sn-Yb.
in (1 0 0) plane, is a vital parameter indirectly affecting the indentation hardness [46] . The highest C44 for PdSnYb indicates that it has the strongest resistance to shear deformation in (1 0 0) plane. The equation (C12-C44) is a classical representation of Cauchy pressure. When the value of Cauchy pressure is positive, it reveals the material is ductile, whereas the negative value represents brittleness [47] . The computed Cauchy pressure for Pd-based intermetallics follows this order: PdSnYb (232 GPa) > PdSn2Yb (93 GPa) > PdSn2Yb (60 GPa) > 0. The largest value of Cauchy pressure for PdSnYb and the smallest one for PdSn2Yb manifest PdSnYb is the most ductile structure and PdSn2Yb is the least one.
For the polycrystalline system, elastic modulus can be got via independent elastic constants. In order to obtain the bulk modulus and shear modulus, we consult the Voigt and Reuss models. Ref. [44] sums up the expressions of bulk and shear modulus for different systems:
For cubic phase (Pd2SnYb):
![]() (6)
(6)
![]() (7)
(7)
![]() (8)
(8)
For orthorhombic phase (PdSnYb and PdSn2Yb):
![]() (9)
(9)
![]() (10)
(10)
![]() (11)
(11)
![]() (12)
(12)
where![]() ,
, ![]() and
and![]() ,
, ![]() , which represent the bulk modulus and shear modulus respectively, are calculated by Voigt and Reuss approximation.
, which represent the bulk modulus and shear modulus respectively, are calculated by Voigt and Reuss approximation.
According to the extreme value principle, the Reuss’s and Voigt’s models have been proved to be the lower and upper limits of the elastic constant by Hill [48] . The formula called Voigt-Reuss-Hill (VRH) agrees well with the experiments:
![]() (13)
(13)
![]() (14)
(14)
where B and G represent the bulk and shear modulus.
The value of bulk modulus and shear modulus, Young’s modulus and Poisson’s ratio using Hill’s models are obtained:
![]() (15)
(15)
![]() (16)
(16)
Melting point, characterizing the thermodynamic stability of alloy, has always been considered as an important parameter. Deduced from Ref. [49] , the melting temperature of materials, which is closely related to elastic constants, is estimated as follows:
![]() (17)
(17)
Table 4 lists the elastic modulus (GPa), bulk modulus (GPa), shear modulus (GPa), Poisson’s ratio ν, Pugh modules ratio ![]() and the melting temperature (˚C) of Pd2SnYb, PdSnYb, and PdSn2Yb.
and the melting temperature (˚C) of Pd2SnYb, PdSnYb, and PdSn2Yb.
Generally, the bulk modulus reflects the average values of bonding strength and the ability to resist volume change. Shear modulus measures the resistance to plastic deformation. As Table 4 shown, the bulk modulus is in the sequence of Pd2SnYb (106 GPa) > PdSnYb (71 GPa) > PdSn2Yb (45 GPa), indicating Pd2SnYb is the least compressible material in all structures. However, the shear moduli of them are almost the same. Young’s modulus serves as a measure of the stiffness. The higher the Young’s modulus is, the stiffer the material will be.
Poisson’s ratio ν and Pugh modules ratio ![]() further confirm the brittleness and ductility of materials. Poisson’s ratio v reflects the elastic parameter of uniaxial deformation, especially in atom binding force. With 0.5 as the critical point, Poisson’s ratio v = 0.5 suggests the constancy of volume. When the variation of v is between 0.25 - 0.5, the atom binding force is central force. The v value
further confirm the brittleness and ductility of materials. Poisson’s ratio v reflects the elastic parameter of uniaxial deformation, especially in atom binding force. With 0.5 as the critical point, Poisson’s ratio v = 0.5 suggests the constancy of volume. When the variation of v is between 0.25 - 0.5, the atom binding force is central force. The v value
![]()
Table 4. The calculated values for elastic modulus (GPa), bulk modulus (GPa), shear modulus (GPa), Poisson’s ratio and Pugh modules ratio![]() , melting temperature Tm (˚C).
, melting temperature Tm (˚C).
for Pd2SnYb, PdSnYb and PdSn2Yb is higher than 0.25, which shows the atomic forces are remarkably central forces. Pd2SnYb presents the largest v, reflecting its resistance of shear strain is the weakest. In accordance with Pugh’s criterion [50] , material with ![]() is brittle; whereas material with
is brittle; whereas material with ![]() shows ductility. In Table 4, the G/B ratios of Pd2SnYb, PdSnYb, and PdSn2Yb are less than 0.57. Thus, these three alloys are deemed to be ductility. The melting point of alloys is implied its thermodynamic stability. In Table 4 the melting temperature of Pd2SnYb, PdSnYb, and PdSn2Yb are 984˚C, 1230˚C, 747˚C, respectively, which further verifies the stability.
shows ductility. In Table 4, the G/B ratios of Pd2SnYb, PdSnYb, and PdSn2Yb are less than 0.57. Thus, these three alloys are deemed to be ductility. The melting point of alloys is implied its thermodynamic stability. In Table 4 the melting temperature of Pd2SnYb, PdSnYb, and PdSn2Yb are 984˚C, 1230˚C, 747˚C, respectively, which further verifies the stability.
3.5. Anisotropy
3.5.1. Elastic Anisotropy
It is well known that single crystal is anisotropic, which has great influence on the performance of thin-film materials. So how to characterize the degree of anisotropic is necessary. To obtain the anisotropic degree of Pd-based alloys, the two-dimensional images of shear modulus of Pd2SnYb, PdSnYb, and PdSn2Yb are described in Figure 3, the two quarter circles with radius of 50 and 100 in
![]()
Figure 3. Two-dimensional graphs of the shear modulus (GPa) in Pd-Sn-Yb alloys. (a) Pd2SnYb, (b) PdYbSn, (c) YbSn2Yb.
Figure 3, which are labeled in black solid line, mean isotropy and play a supporting role in estimating the anisotropic degree.
The share modulus G on different plane along different directions can be expressed as [51] :
(001) plane from [100] to [010]:![]() (18)
(18)
(100) plane from [100] to [010]:![]() (19)
(19)
(010) plane from [100] to [010]:![]() (20)
(20)
(![]() ) plane from [001] to [110]:
) plane from [001] to [110]:
![]() (21)
(21)
(110) plane from [001] to [![]() ]:
]:
![]() (22)
(22)
in which θ represent the angle between [uvw] direction and [HKL] direction.
As we can see in Figure 3(a), the shear modulus of Pd2SnYb in (001), (100), and (010) trajectory planes are similar to the quarter circles, which imply that Pd2SnYb shows almost isotropy in these planes. On the curves of (![]() ) plane from [001] to [110] and (110) plane from [001] to [
) plane from [001] to [110] and (110) plane from [001] to [![]() ], shear modulus show obviously anisotropy of Pd2SnYb. For PdSnYb and PdSn2Yb, shear modulus are all anisotropy due to the noncircular plots on the mentioned planes along different directions. To take a panoramic view of Figure 3, shear modulus is the smallest on (110) plane, which suggests it may be the glide plane of Pd-based alloys.
], shear modulus show obviously anisotropy of Pd2SnYb. For PdSnYb and PdSn2Yb, shear modulus are all anisotropy due to the noncircular plots on the mentioned planes along different directions. To take a panoramic view of Figure 3, shear modulus is the smallest on (110) plane, which suggests it may be the glide plane of Pd-based alloys.
In order to clearly illustrate the anisotropies of mechanical modulus for Pd2SnYb, PdSnYb, and PdSn2Yb, we plot three dimensional surfaces of modulus in Figure 4. For bulk modulus and Young’s modulus, the 3D plots can be more intuitive to determine the ability to withstand external stress. Their formulas are as follow:
bulk modulus [52] :
![]() (23)
(23)
Young’s modulus [53] :
![]() (24)
(24)
where ![]() is the elastic compliance coefficient and
is the elastic compliance coefficient and![]() ,
, ![]() ,
, ![]() ,
, ![]() re- present the directional cosine.
re- present the directional cosine.
In Figure 4, the bulk modulus of Pd2SnYb is spherical, reflecting isotropy of the bulk modulus. The three dimensional graphs of bulk modulus of PdSnYb and PdSn2Yb, and Young’s modulus of Pd2SnYb are irregularly. Thus, they express anisotropic nature, as well as the PdSn2Yb performs the strongest anisotropy. Conversely, Pd2SnYb is isotropy, which is in good agreement with the
![]()
Figure 4. Three-dimensional stereograms of the bulk modulus and Young’s modulus (GPa) of Pd-Sn-Yb.
calculated anisotropy of shear modulus in the present work. Compared with bulk modulus, the projections of Young’s modulus on the (100), (010) and (001) planes show a more pronounced anisotropy. Therefore, a stronger directional dependence of Young’s modulus has displayed on these planes.
3.5.2. Ideal Strength of Tensile and Shear Deformation
It is essential to comprehend the causation of the structural stability for the design and application of these Pd-based alloys, especially the response of lattice stress to the applied strain. To analysis the mechanism of mechanical deformation, the stress-strain curves of tensile and shear deformation are performed in Figure 5.
For tensile deformation, the strain directions [100], [010], [001] are parallel to the coordinate axis of the corresponding unit cell. From Figures 5(a)-(c), the tensile strengths of PdSnYb and PdSn2Yb show anisotropy. And the strongest ideal tensile strengths of these two alloys exist in the strain direction [001]. Due to the different symmetry of Pd2SnYb compared with PdSnYb and PdSn2Yb, it’s isotropic along the strain direction [100], [010], and [001]. The yielding stage of Pd2SnYb, PdSnYb, and PdSn2Yb in different orientations all occurs in 2% strains.
It can be seen in the Figures 5(d)-(f) that the shear moduli can be obtained from the strains less than 2% [54] . On the basis of this linear parts, the computed
![]()
Figure 5. The tensile and shear stress-strain curves of Pd-based alloys along different directions.
shear moduli values are
![]() for Pd2SnYb,
for Pd2SnYb,
![]() ,
, ![]() for PdSnYb and
for PdSnYb and![]() ,
, ![]() ,
, ![]() for PdSn2Yb, respectively. In contrast with the results using Voigt-Reuss-Hill method, they are not accord, which proves that they are all anisotropic in the whole crystal of the three structures.
for PdSn2Yb, respectively. In contrast with the results using Voigt-Reuss-Hill method, they are not accord, which proves that they are all anisotropic in the whole crystal of the three structures.
3.6. The Minimum Value of Thermal Conductivity Kmin
The thermal conductivity is a measure of material’s heat conduction ability. Therefore, the research on it of Pd-based alloys in this work is significant.
Owing to the lattice vibration influences the crystal macroscopic thermodynamic properties, the lattice vibration becomes important factors we want to know. And lattice vibration is determined by phonon system. Thus it has great significance to the materials’ thermal conductivity. The transverse acoustic wave velocity (vt), longitudinal acoustic wave velocity (vl), and wave velocity (vm) are calculated [55] :
![]() (25)
(25)
![]() (26)
(26)
![]() (27)
(27)
In the condition of high temperature, the value of thermal conductivity will decrease with increasing temperature [56] . Hence the minimum thermal conductivity value for materials in the applications of high temperature is extremely important. The minimum thermal conductivity of Pd2SnYb, PdSnYb, and PdSn2Yb is calculated on the basis of Clark’s model [56] and Cahill’s model [57] :
Clark’s Model:![]() (28)
(28)
Cahill’s Model:![]() (29)
(29)
where kB represents Boltzmann’s constant, Ma is the average mass of atoms, E is the Young’s modulus, ρ is density, vn (n = 1, 2, 3) is acoustic wave velocity, p is the number of atoms in unit volume. All the indexes are calculated in Table 5. The thermal conductivity for cubic ZrO2 is also calculated, aiming to compare the value with the experimental value to confirm the accuracy of the calculation method.
As shown in Table 5, the calculated thermal conductivity using the Cahill’s model is lightly greater than that computed by the Clark’s model. This is due to the atom number density and phonon spectrum are both considered in Cahill’s model, whereas the Clark’s model does not [58] . Thus, the Clark’s model underestimates the thermal conductivity, however the value adopting Cahill’s model gets closer to the real values of thermal conductivity. In comparison with Clark’s model, the Cahill’s value of ZrO2 is closer to the experimental value, which confirms this calculation method is credible. As for Pd2SnYb, the minimum thermal conductivity is largest, and that for PdSn2Yb is the smallest. Compared to the results in the present work, the increasing content of Sn atoms cause the decreasing in minimum thermal conductivity, when the proportion of Pd/Sn ratios modify. As is known to all, the Y2O3-stabilized ZrO2 (~2.2 W・m−1・K−1) are investigated for application as materials for thermal barrier coatings. Based on the accuracy of the calculation method, the calculated minimum thermal conductivities of Pd2SnYb, PdSnYb and PdSn2Yb are all at least a quarter less than ZrO2,
![]()
Table 5. Transverse speed vt (km・s−1), longitudinal speed v1 (km・s−1), acoustic speed vm (km・s−1) for Pd-Sn-Yb, and the minimum thermal conductivities Kmin (W・m−1・K−1) of Cahill’s Model, Clark’s Model for Pd-Sn-Yb and ZrO2.
which show Pd2SnYb, PdSnYb and PdSn2Yb can be used for high-temperature- resistant materials, aerospace field, and many other fields.
4. Conclusions
The calculated results showed that the AFM-2 state of Pd2SnYb and the NM state of PdSnYb, PdSn2Yb are found to be the ground state, which are agreed with experimental reports. The obtained enthalpy of formation and binding energy are in the order: 0 > Pd2SnYb > PdSnYb > PdSn2Yb, indicating that the Pd-based alloys are mechanically stable. The Fermi energy (Ef) values of these compounds with Ef (PdSnYb) > Ef (Pd2SnYb) > Ef (PdSn2Yb) imply that PdSnYb is most likely to lose electrons while PdSn2Yb is difficult. In line with the Cauchy pressure, values of Poisson’s ratio ν, and Pugh modules ratio![]() , these three alloys are deemed to be ductility. The three compounds are all elastic anisotropic, and the anisotropic sequence is PdSn2Yb > PdSnYb > Pd2SnYb. The ideal strength of tensile and shear deformation are inconformity in different crystal orientations, implying that Pd2SnYb, Pd2SnYb, and Pd2SnYb are plastic anisotropic. Moreover, the calculated minimum thermal conductivities of Pd2SnYb, PdSnYb and PdSn2Yb are all at least a quarter less than that of ZrO2, the usual thermal barrier coatings materials. That implies these Pd-based alloys can be candidates for high-temperature-resistant materials.
, these three alloys are deemed to be ductility. The three compounds are all elastic anisotropic, and the anisotropic sequence is PdSn2Yb > PdSnYb > Pd2SnYb. The ideal strength of tensile and shear deformation are inconformity in different crystal orientations, implying that Pd2SnYb, Pd2SnYb, and Pd2SnYb are plastic anisotropic. Moreover, the calculated minimum thermal conductivities of Pd2SnYb, PdSnYb and PdSn2Yb are all at least a quarter less than that of ZrO2, the usual thermal barrier coatings materials. That implies these Pd-based alloys can be candidates for high-temperature-resistant materials.
Acknowledgements
This work was supported by Fundamental Research Funds for the Central Universities (XDJK2016D043).