Kinetics Study on Photocatalytic Degradation of Methyl Orange Catalyzed by Sea Urchin-Like Cu2O ()

1. Introduction
Dye wastewater with the characteristics of complex composition, high color and emissions, high toxicity and poor biodegradability has serious polluted the environment. The common treatment methods of the dye wastewater are flocculation precipitation [1], electrolysis [2], adsorption [3], and biological vectors [4] in industry. One common disadvantage of these methods is that they can transfer the contamination from one phase to another rather than being destructive. Therefore, the invention of a new treatment method without secondary pollution is deemed necessary. In recent years, it has become a focus topic that catalyst is utilized to photodegrade the organic pollutants in wastewater. Cuprous oxide (Cu2O) has a direct band gap of 2.0 eV which can be excited by visible light, and it has high stability in solar cells [5]. In the past decade, Cu2O with various morphologies, such as nanosize spheres [6], sea urchin-like [7], porous octahedron [8], nanowires [9] [10], bi-pyra- mids [11] star-like and flower-like [12], has been synthesized by different techniques. Furthermore, it has showed an ideal effect on the photocatalytic degradation organic pollutants in water. However, there are rare reports on the photocatalytic kinetics of Cu2O in previous studies.
In this work, the influence of initial methyl orange (MO) concentration on photo-degradation efficiency was studied. Various kinetics parameters including the reaction rate constants, the activation energy, the pre-expo- nential factor and the reaction order were obtained and the stability property of the sea urchin-like Cu2O was also studied.
2. Experimental
Preparation and characterization of the sea urchin-like Cu2O in detail has been investigated as in [7] and the SEM image of the sea urchin-like Cu2O shows in Figure 1. The sea urchin-like Cu2O of 0.50 g∙L−1 was added into MO solutions with different initial concentrations according to the corresponding proportion. Then the system was illuminated under a 24 W fluorescent lamp (FSL) after the suspension was stirred in darkness for 40 min to ensure adsorption equilibrium. During the reaction, the beaker filled with the suspension was put into a water-bath to maintain the solution at a constant temperature. The distance between the lamp and the solution surface is 15 cm. The suspension was strongly stirred in order to keep the Cu2O well suspended in MO solution. During the course of irradiation, 10 mL of the suspension was drawn once from the mixture solution every 5 min and filtrated the Cu2O. The absorbance of MO aqueous solutions was measured by 7230 G visible spectrophotometer at 464 nm.
3. Results and Discussion
3.1. Effect of Initial MO Concentration on Photodegradation Efficiency
As shown in Figure 2 the degradation rate of MO first increases and then decreases with the increase of MO concentration from 20 mg∙L−1 to 60 mg∙L−1. And the degradation rate of MO after 25 min reaches 90.9%, 92.23%, 94.37%, 91.48% and 35.35%, respectively.
![]()
Figure 1.SEM image of the sea urchin-like Cu2O.
![]()
Figure 2. Effect of different initial MO concentration to the degradation rate.
Compared with diffrentCu2O list in Table 1, sea urchin-like Cu2O showed much higher photocatalytic activity due to the three reasons. (a) Needle-like whiskers increase the surface area that determined by BET method is 3.3961 m2/g. While the BET surface areas of the octahedral morphology and the truncated octahedral morphology are 0.0308 m2/g and 0.1819 m2/g, respectively [13]. (b) The large area exposure of the Cu2O whiskers, in which visible light can occur numerous times of reflection and diffuse reflection, augments the adsorption ability and utilization rate of visible light. (c) The width size of crystal whiskers of the sea urchin-like Cu2O is only about 100 nm, so •OH species formed could easily reach the surface of the crystal whiskers to oxidize MO.
3.2. Reaction Order of the Degradation of MO
The degradation of Cu2O to MO be supposed to first-order kinetics. So, the previous data (before 20 min) taken from Figure 2 were drawn into plots of −ln(C/C0) and irradiation time shown in Figure 3 The results reveal that the photo-degradation conform the pseudo first-order kinetics really.
3.3. The Activation Energy (Ea) and Pre-Exponential Factor (A) of the Sea Urchin-Like Cu2O
The temperature can strongly increase the dye degradation, so the activation energy of degredation be studied by the Langmuir-Hinshelwood model [18].  OH− + h+ → • OH, •OH + MO
OH− + h+ → • OH, •OH + MO 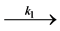 prod-
prod-
ucts. The degradation rate of MO be expressed as: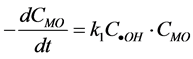 . If irradiation time and the amount of
. If irradiation time and the amount of
Cu2O are constant, the concentrations of •OH radicals are also constant,  , then,
, then,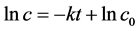 . In
. In
Arrhenius formula: , where, Ea is activation energy and A is pre-exponential factor. In Arrhenius formula:
, where, Ea is activation energy and A is pre-exponential factor. In Arrhenius formula: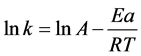 , where, Ea is activation energy and A is pre-exponential factor.
, where, Ea is activation energy and A is pre-exponential factor.
![]()
Table 1. The degradation rate of MO in the presence of different Cu2O.
![]()
Figure 3. First-order decay curve at different initial MO concentrations.
Figure 4 shows the results of lnk at different temperature, lnk are given as: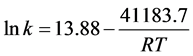 .
.
Ea and A of the degradation are 41.18 KJ∙mol−1 and 1.07 × 106, respectively, with the correlation coefficient of 0.997. Though the raction Ea is little higher than other Cu2O samples [13]-[15] [19]-[21], the significant increase of A also accelerate the reaction, hence the photo-degradation efficiency of MO is much higher than other Cu2O samples [13]-[16].
3.4. Photo-Catalytic Efficiency of Seven Times Recycling Use of Catalyst
Stability of Cu2O was investigated by reuse 7 times shows in Figure 5 It indicates that the efficiency change from 95.84% to 76.1%. The X-ray patterns of residual Cu2O after the seventh recycled, as shown in Figure 6(b), is same as before use. So, the sea urchin-like Cu2O prepared is much stable in the photo-catalysis process under the acidic conditions.
4. Conclusion
The sea urchin-like Cu2O has highly active to degradation of MO under visible light irradiation. Degradation rate was achieved to 94.37% within 25 min. The photo degradation of MO follows the Langmuir-Hinshelwood model and belonging to the first-order reaction. The activation energy and pre-exponential factor are 41.18 KJ∙mol−1 and 1.07 × 106, respectively. After seven times recycling, the Cu2O still showed higher photo-catalytic
![]()
Figure 4. Dependence of the first-order kinetics constant and temperature.
![]()
Figure 5. The degradation to MO of sea urchin-like Cu2O photo-catalyst at different reuse times.
![]()
Figure 6. XRD patterns of sea urchin like (a) before use (b) after reuse for seven times.
efficiency and stability.