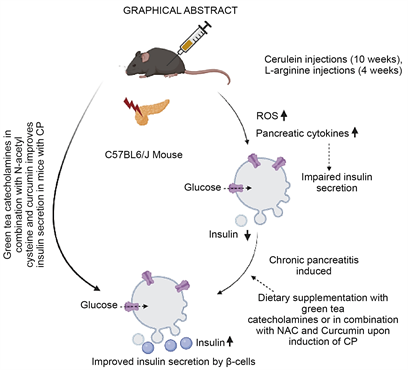
Dietary Green Tea Extract and Antioxidants Improve Insulin Secretory Functions of Pancreatic β-Cells in Mild and Severe Experimental Rodent Model of Chronic Pancreatitis ()
1. Introduction
β-cell dysfunction [1] , and glucose intolerance [2] leading to clinical diabetes are frequent observations during the progression of chronic pancreatitis [3] . Progressive inflammation in chronic pancreatitis culminates in the replacement of pancreatic parenchyma with scar tissue [4] leading to exocrine and endocrine deficiencies [5] . The clinical manifestations include abdominal pain, maldigestion, and diabetes (Type3c diabetes) [6] . This form of diabetes associated with the exocrine disease of the pancreas is ketose resistant, often brittle [7] , and difficult to attain normoglycemia with conventional treatments requiring multiple doses of insulin [8] .
Current treatment aims at pain relief and there are no specific therapeutic strategies for chronic pancreatitis. Although insulin secretory deficiencies [9] were observed in patients with long-standing pancreatitis, the underlying mechanism of progression of insulin secretory response deficiency to clinical diabetes is not studied and no efforts were made to arrest such a progression. Our earlier studies demonstrated that inflammation and altered pancreatic microenvironment in chronic pancreatitis cause islet dysfunction early in the course of the disease and loss of beta cells leads to diabetes late in the advanced stage [10] . We also demonstrated beneficial effects of polyphenolic compound epigallocatechin-3-gallate on islet function in vitro [11] . Therefore, we hypothesized that anti-inflammatory polyphenolic compounds might improve islet functions and delay the progression to clinical diabetes in chronic pancreatitis.
The beneficial effects of green tea are recognized since ancient times and were shown to have anti-inflammatory and anti-oxidative properties [12] [13] [14] . Green tea is obtained from the plant Camellia sinensis. It consists of four catechins, that include epicatechin (EC), epicatechin-3-gallate (ECG), epigallocatechin (EGC), and epigallocatechin-3-gallate (EGCG), in addition to numerous other secondary plant substances. Of these catechins, EGCG is the key bioactive component with medical relevance and has both reactive oxygen species scavenging as well as anti-inflammatory effect.Due to its health benefits, the market for green tea has been growing globally. The economic value of green tea based on the reports by Emergen research is USD 15 billion in 2021 and the demand for green tea is expected to increase further. Studies on cell and animal models clinically reported the health benefits of EGCG, which is the major constituent of green tea [15] [16] . This phytonutrient has beneficial effects on multiple signal transduction pathways related to inflammation [17] , antioxidant [18] , and cell cycle [19] . It is reported to have pleiotropic effects on transmembrane signaling [20] , and remodelling of apolipoprotein amyloid fibrils associated with atherosclerosis [21] . In this context, we hypothesized that green tea may also exert its beneficial effects on the inflammation in chronic pancreatitis. Though EGCG was shown to have multiple health benefits, its role in reversing islet dysfunction associated with chronic pancreatitis has not been focused. The prevalence of idiopathic chronic pancreatitis is high in India (10% - 15% of the total diabetics) [22] , affecting the younger generations with early onset diabetes [23] and management of hyperglycemia is difficult owing to the brittle nature of diabetes secondary to chronic pancreatitis. We therefore wanted to investigate if dietary supplementation of green extract containing EGCG with or without N-acetyl cysteine and curcumin (potential antioxidative compounds with reported role on improving glucose homeostasis) [24] [25] could protect islet functions/reverse islet dysfunction during the progression of chronic pancreatitis. Since it is not feasible to conduct such studies in humans, we have taken up this study in experimental chronic pancreatitis in mice (mild cerulein induced and severe L-arginine induced CP model).
2. Materials and Methods
2.1. Mice Used
C57BL/6J black male mice (25 - 28 g) were maintained at 23 ± 2˚C in sterile standard mice cages with food and water ad libitum. Relative humidity was maintained at 50% - 55% and 12:12 day:night cycle was followed. Body weights of the mice were measured at the baseline and upon completion of the dietary intervention.
2.2. IAEC Approval
Experiments involving mice were carried out following approval and guidelines of institutional animal ethics committee (EGCG/IAEC/01), Animal Research: Reporting of in Vivo experiments (ARRIVE) and National Research Council’s Guide for the care and Use of Laboratory Animals guidelines.
2.3. Induction of Chronic Pancreatitis
Chronic pancreatitis was induced in mice (Cerulein model: n = 102, L-arginine model: n = 30) by repeated intraperitoneal injections of cerulein at a dose of 50 µg/kg every hour for six hours twice a week for 10 weeks in mild model of CP and L-arginine (4.5 g/Kg body weight twice) was injected twice intraperitoneally at hourly interval, followed by two hourly subcutaneous injections of normal saline, once a week till 4 weeks as per the established protocols [26] [27] . Physical activity of the mice was assessed to evaluate any adverse effects of the green tea extract. The pancreata were collected for islet functional studies after induction of chronic pancreatitis and after dietary intervention. Islet functions were assessed by glucose stimulated insulin secretion assay. Histological examinations were conducted to assess the islet morphology and other features of fibrosis.
Mice in cerulein model and L-arginine model were segregated into control, test and dietary intervention group. a. Control group mice (n = 36) received phosphate buffered saline intraperitoneally, b. Test group consisted of mice induced with chronic pancreatitis following cerulein and L-arginine injections for 10 and 4 weeks respectively and mice without dietary intervention for 4 weeks upon induction of cerulein or L-arginine induced CP, c. Dietary intervention group involved mice receiving repeated cerulein injections and supplemented with dietary 0.1% EGCG for 10 weeks; mice supplemented with dietary 0.5% EGCG for 4 weeks upon induction of cerulein or L-arginine induced CP or with 0.5% EGCG, 0.1% N-acetyl cysteine and 0.2% curcumin for 4 weeks upon induction of L-arginine induced chronic pancreatitis (Figure 1 and Figure 2).
Mice with chronic pancreatitis due to cerulein and L-arginine were euthanized after 3 days and post one week of the last cycle of injections respectively. Entire pancreas was carefully dissected and quickly transferred on ice for further
![]()
Figure 1. Flowchart of study design (cerulein model).
processing. The tissue was then divided into two parts; one was fixed in 10% buffered formalin saline for histological examination and the other part excised from the islet enriched gastric lobe was used for islet isolation [28] . After 48 hours of fixation, the tissue was embedded on paraffin and sectioned into 3 μ thick sections for histological studies.
2.4. Rodent Diet
Standard rodent chow
Standard rodent chow [National institute of Nutrition, Tarnaka, Hyderabad, India] containing 27% proteins was fed to mice.
Rodent chow containing green tea extract
Standard rodent chow was supplemented with green tea extract adjusted to a final concentration of 0.1%, 0.2% or 0.5% EGCG.
![]()
Figure 2. Flowchart of study design (L-arginine Model).
Rodent chow containing green tea extract, N-acetyl cysteine and curcumin
Standard rodent chow was supplemented with green tea extract adjusted to a final concentration of 0.5% epigallocatechin gallate, 0.1% N-acetyl cysteine and 0.2% curcumin (Figure 3).
2.5. Mode of Administration of Green Tea in Mice with Chronic Pancreatitis
Mode of administration of green tea extract was assessed by 3 different routes: i) intraperitoneal injections (Dose: 3.125 - 25 mg/Kg body weight); ii) oral gavage (dose: 6.25 - 200 mg/Kg body weight) or iii) through dietary route.
Dietary route: Rodent chow containing green tea extract equivalent to 0.1% EGCG was fed to the mice during the course of disease induction. After establishing the beneficial effects of the dietary green tea extract on islet functions, it was given to mice at 3 different doses, equivalent to 0.1%, 0.2% and 0.5% EGCG after the induction of the disease in cerulein model. Upon optimization of EGCG concentration, further experiments were continued by supplementing mice with rodent chow containing green tea extract equivalent to 0.5% EGCG. Mice in L-arginine model was also tested for islet functions by supplementation of diet with 0.5% EGCG, 0.1% N-acetyl cysteine and 0.2% curcumin [29] .
2.6. Intraperitoneal Glucose Tolerance Test (IPGTT)
Mice were fasted for approximately 16 hours and IPGTT was performed following
![]()
Figure 3. Representative image of various mice groups receiving either standard rodent chow or dietary intervention.
standard protocol [30] [31] . Blood glucose level was measured at the baseline and after one and two hours of glucose injection (i.p) (2 g/Kg body weight), by collecting the blood through tail vein puncture using a glucometer. IPGTT we reperformed before the induction of chronic pancreatitis and after supplementation with green tea extract before the mice were sacrificed.
2.7. Islet Isolation and Glucose Stimulated Insulin Secretion (GSIS)
Resected pancreatic tissues (50 mg) were collected in RPMI 1640 nutrient medium (Sigma Chemicals, St. Louis, MO, USA) and immediately processed for islet isolation employing standard protocols. The pancreatic tissue was minced and subjected to digestion with collagenase V (Sigma Chemicals, USA; 1 mg/ ml in DMEM medium) at 37˚C for 5 - 10 minutes [32] . The resultant dispersed pancreatic islets were washed thrice with RPMI 1640 medium, centrifuged and resuspended in 5 mL of CMRL 1066 medium. Islet cells were stained with dithizone [33] for identification and confirmation (Olympus CX 41; Tokyo, Japan).
Static glucose stimulated insulin secretion was examined in both models of chronic pancreatitis. Islets measuring ~ 150 mm in diameter were handpicked and fifty islet equivalents (IEq) in duplicates were washed initially with RPMI 1640 nutrient medium, followed by washing with Krebs ringer bicarbonate HEPES (KRBH medium; 120 mM NaCl, 5 mM KCl, 2.5 mM CaCl2, 1.1 mM MgCl2, 25 mM NaHCO3, 10 mM HEPES) containing 0.2% BSA. Insulin secretion into the medium by isolated islets in response to basal (2.5 mM) and high (25 mM) glucose concentrations was measured in triplicates by an ELISA method (Mercodia, Uppsala, Sweden) [34] [35] and reported in terms of μg/L insulin released.
2.8. Histological Examination: H&E, Masson’s Trichrome, Picrosirius Red Staining
Paraffin embedded 3 μm thick sections were used for Hematoxylin and Eosin (H&E), Masson’s trichrome staining and Sirius red staining for evaluation of histological features and fibrosis [36] respectively as per standard protocols. All imaging was done using Olympus microscope IX51 model fitted with a camera. For quantification, 10 random fields (10X magnification) per animal were evaluated. The representative images and results reported for H&E staining and Sirius red staining include n = 6 - 8 animals/group.
2.9. Statistical Analysis
Data of all experiments are represented as mean ± SEM. Independent sampled t-test was used to assess the comparison between different groups. A two-sided P-value of <0.05 was considered as statistically significant.
3. Results
3.1. Mice Induced with Chronic Pancreatitis Tolerated Dietary
EGCG
Administration of EGCG either through intraperitoneal or oral gavage resulted in mortality of the mice. However, the mice tolerated EGCG when supplemented as green tea extract (equivalent to 0.1%, 0.2% and 0.5% EGCG) combined with rodent chow. Dietary EGCG containing green tea extract supplementation to the mice did not result in any mortality. Hence, further experiments were conducted by supplementing mice with dietary green tea extract with or without N-acetyl cysteine and curcumin.
3.2. Evaluation of Body Weights of Mice with Cerulein and L-Arginine Induced CP
Induction of CP in both the mice models resulted in significant reduction in body weight in comparison to body weight at the baseline (Cerulein induced CP: 1.2-fold (p value = 0.002), L-arginine induced CP: 1.1-fold, p > 0.0001). Dietary green tea extract supplementation did not result in significant reduction in the body weight of the mice (Figure 4(a) and Figure 4(b)) in CP.
3.3. Morphological Changes in the Resected Pancreas
The pancreas from the sham control mice appeared healthy compared to the reduced parenchyma and fibrous pancreas in mice upon induction of chronic pancreatitis. The extent of reduction in pancreatic parenchyma was more in mice with L-arginine induced CP. Mice pancreas from chronic pancreatitis untreated group showed a slight improvement in the volume of pancreas and it appeared whitish and fluffy, whereas the pancreas from the dietary intervention group had improved morphology compared to the chronic pancreatitis untreated group (Figure 5(a) and Figure 5(b)).
![]() (a) Body weight of mice in cerulein model of chronic pancreatitis
(a) Body weight of mice in cerulein model of chronic pancreatitis![]() (b) Body weight of mice in L-arginine model of chronic pancreatitis
(b) Body weight of mice in L-arginine model of chronic pancreatitis
Figure 4. Body weights of mice before and after dietary intervention in (a) cerulein and (b) L-arginine model of chronic pancreatitis. (EGCG: Epigallocatechin-3-gallate; NAC: N-acetyl cysteine; bwt: body weight ns: P value not Significant; ***P value equal to 0.002; ***P value less than 0.0001).
![]() (a) Cerulein model
(a) Cerulein model![]() (b) L-arginine model
(b) L-arginine model
Figure 5. Morphology of pancreas from mice with CP, CP untreated and with dietary interventions in (a) cerulein and (b) L-arginine mouse model of chronic pancreatitis.
3.4. Histological Examination Reveals Decreased Inflammation and Fibrosis in Green Tea Extract Treated Mice in Cerulein Model but Not in L-Arginine Model of CP
Histological features of pancreatic sections from mice in control, CP induced and EGCG treated group are shown (Figure 6(a), Figure 6(b), Table 1 and Table 2). The acini were largely dispersed in mice induced with chronic pancreatitis and in chronic pancreatitis untreated group, whereas they were observed to be either mildly dispersed or cohesive in the green tea extract supplemented group. Increased ductules were observed in mice induced with CP (cerulein group: 63 ± 5, L-arginine group: 48 ± 2), CP untreated group (cerulein group: 46 ± 4, L-arginine group: 55 ± 5) and they were found to be decreased in mice with CP treated with green tea extract containing 0.5% EGCG (cerulein group: 12 ± 3, L-arginine group: 38 ± 4) or with additional compounds such as N-acetyl cysteine and curcumin (L-arginine group: 20 ± 5).
![]() (a)
(a)![]() (b)
(b)
Figure 6. Histological changes in the resected pancreatic sections of mice upon induction of chronic pancreatitis with and without dietary intervention in (a) cerulein and (b) L-arginine model.
![]()
Table 1. Histological findings in pancreatic sections from mice in cerulein induced CP model with or without dietary intervention.
![]()
Table 2. Histological findings in pancreatic sections from mice in L-arginine induced CP model with or without dietary intervention.
Dietary intervention with 0.5% EGCG containing green tea extract reduced inflammatory cells by 3.4-fold in cerulein CP (1.3-fold and 2.7-fold reduction observed in mice supplemented with 0.1%, 0.2% EGCG containing green tea extract respectively), whereas only 1.3-fold reduction was observed in L-arginine model.
3.5. Glucose Stimulated Insulin Secretion of Islets Decreased in both Mild and Severe Models of Chronic Pancreatitis
Islets isolated from cerulein and L-arginine induced CP mice showed a reduction in glucose stimulated insulin secretion (high glucose challenge) by 76% and 93% respectively in comparison to mice from the sham control group (Figure 7(a)).
Supplementation of dietary green tea extract containing 0.1% EGCG during the course of cerulein injections till 10 weeks showed a 2-fold improvement in insulin secretion upon challenge with high glucose compared to mice without any dietary intervention (Figure 7(b)). However, supplementation of dietary 0.1% for one month after CP induction using cerulein resulted in only 1.1-fold improvement in glucose stimulated insulin secretion in comparison to untreated group.
Further improvement in glucose stimulated insulin secretion by 1.4-fold and 2.1-fold was observed in mice supplemented with increased concentration of dietary EGCG (0.2% and 0.5% respectively) (Figure 7(a)).
In L-arginine induced CP mouse model, dietary 0.5% EGCG containing green tea extract supplementation showed an improved glucose stimulated insulin secretion by 1.6-fold. A further improvement by 3.2-fold was observed upon supplementation of mice with dietary intervention containing 0.5% EGCG, N-acetyl cysteine and curcumin in comparison to the untreated mice with CP (Figure 7(c)).
3.6. Glucose Tolerance Improved with Green Tea Extract Supplementation
In both mild and severe model of chronic pancreatitis, none of the mice were diabetic. The blood glucose levels at 1 hour after glucose challenge in mice with chronic pancreatitis and in mice supplemented with standard rodent chow for 4 weeks upon induction of chronic pancreatitis was higher (Cerulein CP untreated: 0.69-fold increase, L-arginine CP untreated: 0.79-fold increase) compared to the glucose levels at the baseline.
Supplementation with 0.5% dietary EGCG demonstrated lowering of plasma glucose levels at 1 hour (Cerulein CP + 0.5% EGCG supplemented: 1.23-fold decrease, L-arginine CP + 0.5% EGCG supplemented: 1.68-fold increase) and maintenance of glucose homeostasis in comparison with untreated mice (Figures 8(a)-(i)).
![]()
Figure 8. Effect of EGCG on intraperitoneal glucose tolerance test in mice with differentdietary modifications (a - i).
4. Discussion
Diabetes in chronic pancreatitis is distinct and difficult to control because of complex metabolic derangement arising from maldigestion and dysregulated insulin and glucagon response. Islet dysfunction and deficiency in insulin secretory responses were earlier reported in CP patients. We here demonstrate that dietary intervention with EGCG containing green tea extract did not show any adverse effects on the body weight, feed intake and animal activity. In fact, the mice treated with green tea extract were found to be more active than the mice that did not receive any dietary intervention, which can probably be attributed to improved insulin secretion and glucose utilization.
Decrease in the number of inflammatory cells, ductules and vacuoles in the pancreas, indicate that EGCG exerts its anti-inflammatory effect and decreases inflammation in cerulein induced CP in mice. Histological examination demonstrates decrease in the thickness of the collagen fibres in the green tea extract supplemented mice group in cerulein model. Asaumi et al had shown that green tea polyphenol inhibits ethanol induced activation of pancreatic stellate cells which play a major role in pancreatic fibrosis. Such a protective effect of green tea is also recently reported in remodeling apolipoprotein A-1 amyloid fibrils into soluble oligomers in atherosclerosis [37] .
Gradual decrease in the number of inflammatory cells, ductules and vacuoles in the pancreas of mice treated with 0.1, 0.2 and 0.5% EGCG confirms the efficacy of 0.5% EGCG in reducing the inflammation in cerulein induced mild model of CP.
In our initial experiment we isolated islets from mice with cerulein and L-arginine induced chronic pancreatitis, subjected to glucose stimulated insulin secretory functions and could demonstrate functional loss of islets. We found that 0.1% EGCG in the diet improved islet functions up to 2-fold when given along with cerulein injections. This observation demonstrates the proof of concept that anti-inflammatory molecules improve insulin secretion in CP. In the later experiments, green tea extract containing 0.5% EGCG showed improved insulin secretory function of β-cells, glucose homeostasis and reduced inflammation (predominantly consisting of lymphocytes) in mice with cerulein induced CP. The severe model of chronic pancreatitis induced in mice using L-arginine required additional supplementation of N-acetyl cysteine and curcumin along with green tea extract to improve insulin secretory functions in mice with untreated chronic pancreatitis. It also reduced inflammation and improved glucose tolerance proving that dietary anti-inflammatory and anti-oxidative supplementation can reverse islet dysfunction during progression of the disease as well as after the induction of CP. These observations demonstrate the therapeutic potential of green tea extract containing EGCG and anti-oxidative compounds in preserving functions of reminiscent β-cell mass under in vivo conditions in mice.
Lowering of plasma glucose levels after green tea extract supplementation also demonstrate that anti-inflammatory and antioxidant properties of green tea can maintain glucose homeostasis. Our results are in corroboration with earlier studies demonstrating beneficial effects of green tea in improving insulin resistance in Hepg2 cell line and in diabetic complications such as nephropathy, cardiomyopathy etc. Although improved insulin secretion, improved pancreatic parenchyma and reduced inflammatory cell infiltration was observed in the dietary intervention mice pancreas, adiposity was also observed in mice supplemented with dietary intervention containing green tea extract, N-acetyl cysteine and curcumin. Further studies are therefore imperative to develop better dietary combinations to reduce adiposity and aid in preventing adipokine induced damage to the pancreas and to regenerate pancreatic parenchyma.
In conclusion our results demonstrate the therapeutic, anti-oxidative and anti- inflammatory potential of the green tea extract (containing EGCG as the major catechin) alone or in combination with antioxidative compounds like N-acetyl cysteine and curcumin to improve beta cell functions in chemically induced chronic pancreatitis in mice.
Data Availability
Data generated during this study will be made available upon request to the corresponding author.
Declaration of Generative AI in Scientific Writing
The authors did not use AI or AI-assistance either in writing or to draw insights from the data.
Statement of Ethics
The study on mice was approved by the Institutional animal ethics committee of Asian Healthcare Foundation (EGCG/IAEC/01).
Funding
The current study was funded by Indian Council of Medical Research (ICMR- BMS/ADHOC/CMB/2015-2792/JUN-16/2/AP/PVT) and partly by Asian Healthcare Foundation.
CRediT Authorship Contribution Statement
Sheethal Galande: Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing—original draft, Writing—review & editing, Validation. Ranjeet Tokala: Data curation, Methodology, Resources, Writing—review & editing. Pavan Pondugala: Investigation, Methodology, Visualization. Krishna Vemula: Methodology, Validation. Vijayalakshmi Venkatesan: Conceptualization, Resources. Pothani Suresh: Resources. Surya Satyanarayana Singh: Writing—review & editing. G V Rao: Resources, Writing—review & editing. D Nageshwar Reddy: Resources, Writing—review & editing. Mitnala Sasikala: Conceptualization, Formal analysis, Visualization, Resources, Writing—review & editing.
Acknowledgements
The authors thank AIG Hospitals, Gachibowli, Hyderabad, India, for providing the infrastructure required to conduct this research and Dr. Rupjyoti Talukdar (MD, AIG Hospitals) for providing his critical insights in this study.