Effects of Curing Conditions and Formulations on Residual Monomer Contents and Temperature Increase of a Model UV Gel Nail Formulation ()
1. Introduction
Recently, ultraviolet (UV) curable monomers (also known as UV gel nails) have become a popular decoration technique for women’s nails. Curing (polymerizing) UV gel nails results in the formation of a harder, glossier, more attractive, and durable solid polymer layer than conventional manicures. Although the UV gel nail market is flourishing and has expanded rapidly, safety guidelines on UV gel nail treatment have not yet been established. Clinical studies on UV gel nails have revealed that technicians at nail salons are at risk of developing allergies [1-3] and asthma [4,5] due to UV gel nails. Some UV-curable monomers may cause human skin allergies and asthma in respiratory organs. Most clinical studies have focused on the relationship between the monomer and the human body although dental composite resin, which is applied using photopolymerization, has also been studied to determine the residuals of the cured composites [6-10]. The quantity of monomers remaining in the cured UV gel nails has not been determined yet. To determine the risk the monomers pose to UV gel nail technicians and salon customers, the amount of residuals (monomer, photoinitiator and stabilizer) that leak from cured UV gel nails must be evaluated. Currently, UV gel nail kits are sold in commodity stores. People who are not knowledgeable of the risk that monomers and UV light pose can buy the kit and apply the nails by themselves, which increases the severity of the situation.
The UV gel nail treatment procedure can be divided into several steps: 1) filing the human nail; 2) applying the UV gel nail onto the human nail; 3) exposing the UV gel nail to UV light to polymerize the UV curable monomers; and 4) wiping the unreacted monomers from the cured UV gel nail with a tissue.
UV curable monomers, which are the raw materials used in UV gel nails, have been used in industrial coating processes; they require high intensity UV light and inert (nitrogen or carbon dioxide) atmospheres [11]. However, for UV gel nails, the intensity of UV light should be lower (approximately 3 - 10 mW/cm2), than that used for the industrial processes to avoid unexpected heating. Moreover, inert atmospheres, which can cause asphyxia, cannot be used in UV gel nail salons. Thus, most UV gel nails are cured in air. When a UV-curable monomer is cured in air in the presence of low-intensity UV light, the UV-curable monomers do not achieve 100% conversion (molecular base) due to oxygen-induced inhibition of polymerization. Thus, an unreacted layer forms on the surface of the cured film and residual monomers exist in the cured film, which must be removed by wiping the surface with a tissue that has been moistened with ethanol. Residual monomers can travel from the cured resin and may cause problems in the human body as has been indicated by clinical studies.
In addition to the residual monomer contents, temperatures observed during the UV curing of monomers should be maintained within the range of human body temperature. UV gel nails use radical polymerizations, which are exothermic chemical reactions. The UV curing reaction is rapid enough (one minute) that heat does not dissipate from the nail, which increases the temperature of the human nail. A rapid temperature increase may occur when the intensity of UV light is too high or the gel nail is too thick. Although the temperature is not a crucial parameter for industrial processes, temperature changes during polymerization should be considered for human nails.
In the present study, the thickness of the unreacted layer, the temperature increase, and the residual content of a model UV gel nail formulation were measured. The thickness of the unreacted layer and the residual contents were quantitatively determined using the gravimetric method and chromatography. The temperature profile and the maximum temperature of the UV curing process were recorded under UV exposure of the UV gel nail. Finally, practical ranges of cast thickness for the UV gel nail, UV light intensities, and exposure times were identified.
2. Materials and Methods
2.1. Materials
Diurethane dimethacrylate (436909, 225 ppm ± 25 ppm topanol as inhibitor, Aldrich, St. Louis, MO), 1-hydroxycyclohexyl phenyl ketone (Irgacure 184, BASF, Ludwigshafen, Germany), and diethyl amine (Wako Pure Chemical Industries, Osaka, Japan) were used as the UV curable monomer, the photoinitiator and the stabilizer, respectively. Diurethane dimethacrylate is a popular material for UV gel nails and can be used alone or as a mixture with reactive diluents. The monomer, photoinitiator, and stabilizer were mixed in a 50 ml light-shielded polypropylene bottle with a mechanical agitator for 30 min at room temperature and were stored at 40˚C overnight. Sample solutions containing 1% or 5% of the photoinitiator were prepared, and 0.2% of diethylamine was added to the solutions. The photoinitiator was completely dissolved in the monomer.
2.2. UV Curing and Extraction
Using a frame applicator (Imoto Machinery, Kyoto, Japan) operated at 30 cm/min, the sample solution was applied to a polyester substrate (100 μm in thick, OHP- 10CN, OHP sheet, Tochiman, Japan), which was used as an alternative to human nails. The cast film was cut into 3 cm ´ 3 cm pieces and was weighed using an electronic balance (minimum readout = 0.01 mg, Shimadzu, Kyoto, Japan). Without the polyester substrate, the typical mass of a cast film with a thickness of 100 mm was approximately 100 mg. The cast thickness of the sample, L0, was calculated according to the following equation:
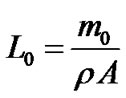 (1)
(1)
where m0 is the mass of the cast sample without the polyester substrate, ρ is the density of the sample solution and A is the cast area. The density of the sample solution (1.1 g/cm3) was obtained from the supplier of the monomer.
Figure 1 shows a schematic diagram of the simultaneous UV exposure and temperature monitoring apparatus. The cast film was placed on a stage, and UV light was guided from a high pressure 200 Watt Mercury Vapor Short Arc lump (OmniCure S2000, ExFo, Quebec, Canada) to the sample stage.
The typical 4 cm distance between the cast film and the UV light could be adjusted to change the intensity of the UV light. The intensity of the UV light at the center of the film was set to the maximum and was attenuated concentrically. The cast film was exposed to UV light in an atmosphere of air at the desired intensity, 3 mW/cm2.
Prior to UV exposure, the intensity of the UV light at the center of the film was monitored using a UV meter (Ushio Unimeter UIT-150, Ushio, Japan) and was adjusted to achieve a desired intensity. The intensity of the UV light and the exposure time of the typical curing conditions, which was measured with a commercial UV gel nail lamp, were 3 mW/cm2 and 60 s, respectively. The intensity of light and the exposure time were varied to investigate their effects on the UV gel nail curing process. The UV exposure experiments were repeated three
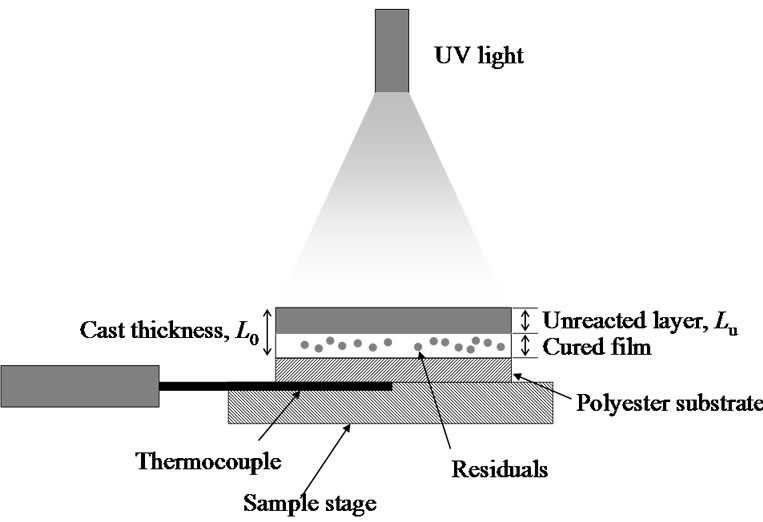
Figure 1. Schematic diagram of the simultaneous UV exposure and temperature monitoring apparatus. The sample solution was cast on a polyester substrate on a sample stage, and the solution was exposed to UV light. A cured film, an unreacted layer and some residual monomer were formed. The temperature increase was monitored using a thermocouple. The figure is not drawn to scale.
times at each condition to assess the reproducibility.
After UV exposure, a tacky unreacted layer usually formed on the cured film. The unreacted layer was removed by wiping with tissue (KimWipes, KimberlyClark, USA) that had been moistened with ethanol (purity = 99%, Wako Pure Chemicals Industries, Osaka, Japan) to remove unreacted monomers on the surface of the cured film. The wiping was performed by hand and was repeated at least three times. After wiping, films were no longer tacky.
This procedure is commonly performed in UV gel nail salons. We used this procedure to determine the amount of residual monomer in the cured layer that could not be removed by wiping. The thickness of the unreacted layer, Lu, was calculated according to the following equation:
 (2)
(2)
where mw is the mass of the wiped film.
The wiped film was placed in a beaker filled with 20 ml of ethanol and was sonicated for 30 minutes at room temperature to extract the residual monomers, the photoinitiator and the stabilizer from the cured film. Subsequently, the film was dried in an oven at 90˚C for 1 hour and was cooled to room temperature. The mass fraction of the extracted residuals, fe, was calculated according to the following equation:
 (3)
(3)
where me is the mass of extracted film.
2.3. GPC Analysis
Gel permeation chromatography (GPC) was used to determine the amount of monomer in the post-sonication ethanol solution. In total, 10 μL of the extracted solution containing the extracted residuals was injected into a GPC. A series of Shodex K806L and K-800D (Showa Denko, Tokyo, Japan) columns were heated to 40˚C. High-pressure liquid chromatography-grade chloroform (Wako Pure Chemicals Industries, Osaka, Japan) was used as the eluent, and a UV-Vis spectrometer (254 nm, Shimadzu, Kyoto, Japan) was used as the detector.
2.4. Temperature Increase
To evaluate the temperature increase during the UV curing process, a K-type thermocouple (0.5 mm in diameter) was placed below the polyester film as shown in Figure 1. The heat of photopolymerization during the UV curing reaction caused the temperature of the sample to increase and caused the heat to conduct through the polyester film. The temperature of the film was recorded every 0.167 s.
3. Results and Discussion
3.1. Temperature Increase
Figure 2 shows the temperature profile of the UV curing reaction. The photoinitiator concentration, the light intensity, the film thickness and the curing time were 1%, 3 mW/cm2, 100 μm and 60 s, respectively. When UV light was applied to the film, the temperature increased dramatically from 23.2˚C to 29.2˚C.
After the maximum temperature was observed, the temperature decreased over time. When the UV light was turned off, the temperature continued to decreases and approached room temperature asymptotically. Two temperature ranges were observed during the UV-curing reaction. The first temperature range was associated with the heat of photopolymerization and the absorbance of UV light by the sample. The second temperature range was attributed to the heat of polymerization. Using the maximum temperature, a temperature index of the profile was obtained and was employed thereafter.
3.2. GPC Analysis
To determine the amount of residuals in the cured film, the wiped film was sonicated in ethanol. Residuals were extracted into ethanol, and the solution was injected onto the GPC column to analyze the residual monomer contents.
Figure 3 shows the GPC profile of the extracted solution and the monomer. The monomer peak was observed at 12.4 min, and the extracted solution showed two distinct peaks. The earliest peak of the chromatograph of the