A New Schiff Base Derivatives Designed to Bind Metal Ion (Cu, Co): Thermodynamics and Biological Activity Studies ()
Received 26 March 2016; accepted 10 May 2016; published 13 May 2016

1. Introduction
Schiff base derived from an amine and carbonyl compound is an important class of ligands that coordinate to metal ions via azomethine nitrogen and has been studied extensively [1] . The characterization bond of Schiff base (C=N) has reversible nature which allows by hydrolysis, obtaining the initial corresponding aldehyde and amine compound [2] [3] . Schiff bases are known to be good chelating agents and easily prepared and characterized. Little interest has been given to their use for analytical purpose because of two serious drawbacks:
1) They are insoluble in aqueous solution.
2) They decompose easily in acidic solution limiting their use to basic solution [4] .
The chemistry of (C=N) double bond based complexes plays a vital role in the progress of chemistry and it has been found to possess the pharmacological activities such as malarial [5] , anticancer [6] , antibacterial [7] . Schiff base appears to be an important intermediate in a number of enzymatic reactions involving interaction of enzyme with an amino or carbonyl group of substitutes [8] . The aim of the study is the preparation of Schiff base derived from salicylaldehyde with a series of aniline derivatives. The structure of all Schiff bases was characterized by using FTIR spectra, CHN elemental analysis and UV-Visible. Their stability constant and their thermodynamic parameters such as (∆E, ∆G, ∆H, and ∆S) were studied in different temperatures spectrophotometrically.
2. Experimental
2.1. Material and Instrumentation
All chemicals and solvents were purchased from Fluka and BDH chemical companies. Cobalt and copper acetate were dried over P4O10 under vacuum for several days before use. Melting point was recorded on VeeGO Digital model VMP-D. Jenway. Elemental analyses (CHN) were performed by Perkin Elemer TGA. IR spectra used in the characterization of products were recorded Shimadzu, FTIR-8400S-JAPAN. UV/Vis absorption studies were measured in the region (200 - 800) nm using (10−4) M solution in ethanolic solution using U.V-9200 BIOTECH ENGINEERN MANAGEMENT CO. LTD (UK) Single beam.
2.2. Preparation of Ligands
The Schiff base was synthesized by refluxing ethanolic solution of (2.44 gm - 0.02 mol) salicylaldehyde with (2.74 gm - 0.02 mol) of substituted (o-benzoic, m-benzoic, p-benzoic) aniline in 20 ml warm ethanolic solution (40˚C - 50˚C, to speed up the reaction), the ratio is (1:1) for two hours [9] . The ligand formed was filtered, washed, recrystallized from ethanol and then dried.
2.3. Preparation of Schiff Base Metal Complexes
An ethanolic solution of (2 × 10−2 M) Schiff base was added to (10−2 M) of metal ions (as dehydrated acetate salts). The mixture was stirred and refluxed for two hours. The solid coloured product obtained was filtered, washed and recrystallized from ethanol and then dried.
3. Result and Discussion
3.1. Physical Measurement
Physical measurements of the synthesized ligand and their metal complexes and their expected formula are shown in Table 1. Melting point gives an impression that the synthesis from Schiff base and their complexes are without any contaminated material.
![]()
Table 1. Physical properties of synthesized ligand and their metal complexes.
L1 = o - position, L2 = m - position, L3 = p - position.
3.2. The Elemental Analyses (CHN) of Ligand and Their Metal Complexes
The data of element analysis (CHN) have shown that the results obtained are in good agreement with those calculated for suggested formula (Table 2).
3.3. IR Spectrum of Ligand and Their Ligand Metal Complexes
Table 3 shows the absence of band at 1735 cm−1 (C=O) due to carbonyl and 3315 cm−1 for NH2 stretching vibration of strong new band appeared at 1630 cm−1 assigned to azomethine (HC=N) which indicates that amine and aldehyde of starting material are absent and have been converted into the new ligand. The IR spectra of complexes exhibited ligand bands with the appropriate Shifts due to complex formation (C=N) at (1630 cm−1) in free ligand shift to (1620 cm−1). The reduction in band order upon complexation can be attributed to delocalization of metal electron density to the π-system of the ligand. These Shifts confirm the coordination of the ligand via nitrogen of the azomethine to the metal ions (Figure 1). All lower frequency of complexes exhibited band around (400 - 600 cm−1) assigned to the υ (M-N) [10] [11] .
3.4. UV-Visible Spectrum of Ligand and Their Metal Complexes
Spectrophotometrically from the wide studied range of molar concentration (10−5 - 10−3 M) of the mixed solution only concentration of (10−4 M) obey Beer-lambert law and showed intense colour, A calibration curve was plotted on absorbance against molar concentration in the range of (1 × 10−4 - 3 × 10−4 M), best fit straight line, were obtained. The composition of complexes formed in solution has been established by mole ratio and continuous variation methods, in both cases the result reveals (1:2) (M:L) ratio.
![]()
Table 2. Elemental analysis data for schiff bases and their complexes.
L1= o - position, L2 = m - position, L3 = p - position.
![]()
Table 3. IR spectra of Schiff base and its metal complexes.
L1 = o - position, L2 = m - position, L3 = p - position.
3.5. Determination the Stability Constant for Complexes
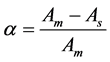 (1)
(1)
where α = degree of analysis [12] [13] , Am = absorbance of equivalent amount of L:M, As = absorbance of excess amount of ligand with constant volume of metal.
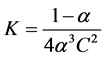 (2)
(2)
where C = concentration of the ligand which is equal the concentration of the metal.
3.6. The Thermodynamic Parameters
The thermodynamic parameters (Table 4) were calculated from their stability constant at different temperatures such as (∆G, ∆H and ∆S) from the following equation [14] [15] :
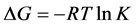 (3)
(3)
 (4)
(4)
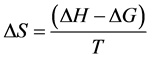 (5)
(5)
where, K = Stability Constant, G = Free Energy, H = Enthalpy, S = Entropy.
3.7. Biological Activity
The biological activity were studied, the effect of schiff base derivatives and its complexes on two type of
![]()
Figure 1. Suggest structure of metal―complexes Schiff base.
![]()
Table 4. Shows the difference stability at different temperature and then thermodynamic parameter was recorded.
![]()
Table 5. The biological activity of ligand and their metal complexes.
L1 = o - position, L2 = m - position, L3 = p - position.
Bacteria Aeromomas hydrophila (−) and Staphylococcus aureus (+) have been described in Table 5. The result show that the complexes have more toxicity against the bacterial species than free ligand. This can be attributed to the tweeds chelation theory [16] according to which the chelation reduces the polarity of metal ions mainly because of the partial sharing of its positive charge with donor group and possible electro delocalization over the whole ring.
4. Conclusion
Schiff base of [o-benzyl, m-benzyl, p-benzyl] aniline with salisaladehyed was synthesized and characterized by analytical and spectral, FTIR, CHN technique. These compounds exhibited significant biological activities.
Acknowledgements
The author thanks the Iraqi Government, Ministry of Higher Education and Basrah University for the financial support provided for this work. The author is thankful of the support of the college of Education and Iraq Marine Science Center, Basrah University in undertaking the antibacterial studies.