Evaluation of the Performance of Two Selective Enrichment Media and Two Selective Plating Media for the Detection of Salmonella from Primary Poultry Production, According to ISO 6579:2002 ()
1. Introduction
The poultry production settings have been frequently implicated as major reservoirs for Salmonella species, and Salmonellas still remains a major problematic zoonosis resulting in significant morbidity in both humans and animals worldwide [1] [2] . Zoonotic Salmonellas are the most frequently identified agents causing gastroenteritis in humans and animals worldwide and are responsible for the significant morbidity and mortality in humans and animals resulting in an estimated 1.3 billion cases with approximately 3 million deaths [2] [3] . Human salmonellosis cases have been frequently traced to be due to the consumption of contaminated foods of animal origin, especially poultry and poultry products [4] - [6] . In order therefore to prevent and ultimately eliminate food borne salmonellosis, it would be of great importance to put in place surveillance and monitoring programs for food safety.
Food safety is mainly geared towards the detection of pathogenic microorganisms that may render the food unsuitable for human consumption and the conventional methods for the detection of Salmonella and other food borne pathogens is by the traditional culture methods. These methods have the merits of reliability of the media, easy to perform and the capacity to actually recover the causative agents even at very small numbers [6] [7] . The increasing application of external quality assurance programmes has led to wide use of international standard methods, such as International Organization for Standardization (ISO) 6579:2002 intended for foodstuffs and animal feed stuffs. In recent years, the ISO 6579:2002 standard method for detection of Salmonella from primary animal production has been evaluated and widely adopted by several standard laboratories worldwide [8] . This standard method requires four relevant stages which involves 1) non-selective pre-enrichment in modified Buffered Peptone water (BPW); 2) the selective enrichment in Modified Semisolid Rappaport-Vassiliadis (MSRV) or Muller-Kauffmann tetrathionate-novobiocin broth (MKTTn); 3) the selective plating on xylose-ly- sine-deoxycholate (XLD) agar and an additional suitable selective plate of choice and; 4) confirmation characteristics [5] [9] - [12] .
Previously, the Rappaport-Vassiliadis (RV) enrichment broth was modified to the Rappaport-Vassiliadis Soy (RVS) broth which has currently been further modified into the Modified Semisolid Rappaport-Vassiliadis (MSRV). These amendments were aimed at heightening the reliability of the enrichment medium [12] [13] . The MKTT enrichment broth was the first to develop by Muller andwas later supplemented with Ox bile and Brilliant Green in order to enhance selectivity by Kauffmann and Jeffries further included the addition of novobiocin at 40 mg/L in order to inhibit indigenous flora, such as Proteus spp., and henceimproving on the rate of recovery of Salmonella [14] - [16] . Two selective solid media are required for the isolation of Salmonella following selective enrichment. The first preferred medium is xylose lysine deoxycholate agar (XLD) and the second can be any other equivalent selective medium capable of recovering lactose-positive Salmonella, S. Typhi, and S. Paratyphi strains. These may include Hektoen enteric (HE) agar, bismuth sulfite (BS) agar, and brilliant green (BG) agar etc. [17] [18] .
The detection of Salmonella using traditional selective plating media is based on lactose fermentation and hydrogen sulfide (H2S) production by the organism. However, most media have poor specificity, creating an abundance of false positives and resulting in time-consuming identification [19] . Therefore, to reduce the workload for unnecessary examination of presumptive colonies, and to enable the fast and easy detection of Salmonella, it is of important necessity to investigate which selective plating media will provide results that are closest to the true situation. There are a good number of selective plating media that have been developed on the basis of the biochemical characteristics of Salmonella, such as α-galactosidase activity in the absence of β-galactosi- dase activity, C8-esterase activity, catabolism of glucuronate, glycerol, and propylene glycol, andhydrolysis of X-5-gal [19] [20] . In spite of the numerous studies that have aimed at determining the optimal selective media showing high level of sensitivity and specificity for the detection of Salmonella in food and environmental samples [12] , this current study was aimed at evaluating the sensitivity and specificity of Brilliant Green (BG) selective plating medium compared with the preferential selective plating medium, Xylose lysine deoxicholate (XLD). This study determined the rate of recovery of Salmonella from the primary production of poultry and compared the sensitivity, specificity, and the rates of false positives and false negatives of the selective enrichment media (MSRV and MKTTn) and the selective plating media (XLD and BG) for the detection of Salmonella according to ISO 6579:2002.
2. Materials and Methods
2.1. Sample Collection
From August 2013 and May 2014, three hundred and seventy four (374) samples were randomly collected from three different sources in the poultry production settings within Calabar, Nigeria: out of which 170 were obtained from the poultry environmental sources (poultry feeds, poultry drinking water, poultry litter, poultry abattoir reins and dust from poultry house), 136 were obtained from poultry birds themselves (guts, cloacal swabs, poultry meat and eggs) and 68 were obtained from the poultry personnel (poultry personnel stool and hand washings). The samples were stored in an ice chest container and transported to the laboratory for immediate processing. Samples were collected based on the method as described by Akond et al. [21] .
2.2. Isolation of Salmonella Species
The isolation of Salmonella species from the poultry production setting in Calabar was based on the ISO 6579:2002 involving the non-selective pre-enrichment stage using 10% buffered Peptone Water (BPW), the selective enrichment stage using Modified Semisolid Rapapport-Vassiliadis (MSRV) and Muller-Kauffmann Tetrathionate-nivobiocin (MKTTn) selective enrichment media, and finally the selective plating stage using Xylulose Lysine Deoxycholate (XLD) agar and Brilliant Green (BG) selective plating media.
A dilution of 1 in 10 of the samples were prepared in modified buffered peptone water (BPW) and incubated over night at 37˚C. 1 ml and 0.1 ml of the non-selective enrichment (modified BPW) was inoculated into 9 ml of MKTTn and MSRV selective enrichment media respectively. These were respectively incubated at 37˚C and 42˚C in separate incubators for 24 h. After incubation, a loop full from each of the selective enrichment media was streaked onto both Xylose Lysine Desoxycholate (XLD) and Brilliant Green (BG) selective plates.
2.3. Confirmation of Salmonella Isolates
Presumptive Salmonella positive colonies on selective plates were picked by means of a sterile wire loop and used to stab and streak on pre-prepared Triple Sugar Iron Agar (TSIA) and Christensen agar (CA) slants. Colonies that produced alkaline slope/acid butt with or with out the production of H2S and gas, on TSIA slant and Urease negative on Christensen agar slant were considered suggestive of Salmonella. Further confirmation was based on the standard biochemical techniques involving Lycine Decarboxilation (LCD) test, β-alactosidase test, Acetone production test and Indole production test. Finally confirmation was supplemented by serological confirmation involving commercially available polyvalent Salmonella antisera kit (Denka Seiken Co. Ltd. Tokyo, Japan) specific for all group and type-factor Salmonella antigens.
2.4. Statistical Analysis
The data generated in this study were analysed by means of the Statistical Package for Social Sciences (SPSS) 20.0 (IBM, USA). The prevalence of Salmonella from the primary production of poultry from the three sources as obtained by the two selective plating media (XLD and BG) were analysed by means of the Fisher’s exact test. P-values of less than 0.05 (P < 0.05) was considered statistically significant.
Selective plating media exhibiting Salmonella colonies was considered presumptive Salmonella positive plates prior to confirmation. Presumptive Salmonella positive plates that showed positive Salmonella colonies following confirmation were considered as true positives and the others as false positive. Among the selective plating media that did not reveal Salmonella colonies, those that demonstrated Salmonella colonies following confirmation were considered false negative and the others true negatives. The Sensitivity (Sc), Specificity (Sp), positive predictive value (PPV) and negative predictive value (NPV) were also analysed according to the study carried out by Carrique-Mas et al. [22] . The Sensitivity (Sc) was given as the proportion (0 ≤ Sc ≤ 1) of the confirmed Salmonella positive plates that came from the presumptive Salmonella positive plates, while the Specificity (Sp) was given as the proportion (0 ≤ Sp ≤ 1) of the confirmed negative plates that did not show presumptive Salmonella colonies. The PPV and NPV was given as the proportion (0 ≤ PPV ≤ 1 and 0 ≤ NPV ≤ 1) of the positive Salmonella selective plates and negative selective plates that were confirmed as positive Salmonella selective plates and negative selective plates respectively.
3. Results
Out of 374 samples obtained from the poultry production setting in Calabar, 221 (59.1%) were confirmed to be positive for Salmonella species as determined by the biochemical and serological confirmation characteristics (Table 1).
Table 2 shows the number of confirmed Salmonella positive platesfor the recovery of Salmonella species from MSRV and MKTTn selective enrichment media with XLD and BG selective plating media. Fewer confirmed Salmonella positive plates were obtained from XLD and BG plating media when MKTTn, rather than when MSRV enrichment medium was used. However, the difference was not statistically significant (P-value > 0.05). When XLD selective plating media was used, samples cultured in MSRV yielded 217 (98.2%) confirmed Salmonella positive plates while samples cultured in MKTTn yielded 199 (90.0%) confirmed Salmonella positive plates. When BG selective plating media was used, samples cultured in MSRV yielded 211 (95.5%) confirmed Salmonella positive plates while samples cultured in MKTTn yielded 142 (64.3%) confirmed Salmonella positive plates.
Table 3 shows the comparison of the test parameters (sensitivity, specificity, PPV and NPV) for the recovery of Salmonella species from samples following culture on selective plating media. The combination of MSRV/ XLD demonstrated the highest sensitivity (0.98), followed by MSRV/BG (0.95), MKTTn/XLD (0.90) and MKTTn/BG (0.71), while the highest specificity was observed in the combination of MSRV/BG (0.88) followed by MKTTn/BG (0.65), MSRV/XLD (0.50) and MKTTn/XLD (0.13). The PPV of presumptive Salmonella positive plates using MSRV/BG combinations was higher (0.92) (not significantly high) than any other combinations. The NPV of negative plates using MSRV/XLD (0.95) and MSRV/BG (0.93) combinations were higher (significantly high) than the combinations of MKTTn/XLD (0.48) and MKTTn/BG (0.63). The number of false positive XLD plates was higher than the number of false positive BG plates, particularly in the case of MKTTn/
![]()
Table 1. Detection of Salmonella from the primary production of poultry.
![]()
Table 2. Positivity of selective plating media in combination with selective enrichment media.
a = there was no statistical significant difference among the all the combinations of selective enrichments media with selective plates. MSRV/XLD = Xylose lysine deoxycholate agar in combination with Modified semisolid rappaport-vassiliadis enrichment medium; MSRV/BG = Brilliant green agar in combination with Modified semisolid rappaport-vassiliadis enrichment medium; MKTTn/XLD = Xylose lysine deoxycholate agar plate in combination with Muller-Kaufmann tertrathionate novobiocin; MKTTn/BG = Brilliant green agar plate in combination with Muller-Kaufmann tertrathionate novobiocin.
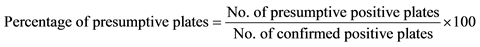
![]()
Table 3. Comparison of the selective plating media for the detection of Salmonella from enrichment media.
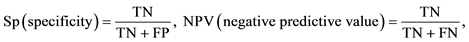
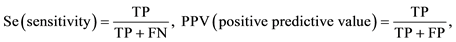 TP = True Positive, FP = False Positive, TN = True Negative, FN = False Negative. The sensitivity and specificity of BG plating media (0.95 and 0.88 respectively) as demonstrated in this study was better than XLD plating media (0.98 and 0.50) when MSRV selective enrichment media was used.
TP = True Positive, FP = False Positive, TN = True Negative, FN = False Negative. The sensitivity and specificity of BG plating media (0.95 and 0.88 respectively) as demonstrated in this study was better than XLD plating media (0.98 and 0.50) when MSRV selective enrichment media was used.
XLD combinations (133 false positive plates). The number of false negative BG plates was higher than the number of false negative XLD plates, particularly in the MKTTn/BG combinations (59 false negative plates).
4. Discussion
The recovery of Salmonella species from the primary production of poultry demonstrated a rate of 59.1%. Several countries have recorded different rates of recovery of Salmonella species from poultry settings such as 70.5% in Brazil [23] , 63.6% in Ethiopia [24] , 53.3% in Vietnam [25] , 47.7% in Austria [26] , 42.3% in Korea [11] , 17% in USA [27] , 10.1% in Georgia [28] and 1% in Jamaica [29] . Such varying rates of recovery of Salmonella can be attributed to differences in the geographical location, the standards of hygiene and sanitation practices observed in these regions, in addition to the methods used in the detection of Salmonella.
The rate of recovery of Salmonella species from the poultry production setting in this current study was higher compared to a previous study carried out in 2009 by Garba et al. in Yola, Nigeria, which recorded a 40.8% rate of recovery of Salmonella species [30] . This result suggests an increasing trend of Salmonella in the poultry production system in Nigeria in addition to poor hygiene/sanitation practices in the primary poultry production settings in Nigeria. This has a great implication in that it poses a serious threat in terms of veterinary health which can lead to the loss of a greater majority of poultry birds and most importantly to the consumer population who may become infected. Moreover, this is in agreement with the report of Garba et al. (2010), who diagnosed more than 50% of the death of birds in Yola, Nigeria to be caused by Salmonella species [30] , meanwhile several studies from different parts of the world have incriminated poultry and poultry products as the major source of salmonellosis in humans [6] .
This current study was aimed at assessing the performance of XLD as preferential selective plating medium by comparing it with BG for the detection of salmonella in the primary production of poultry based on the ISO 6579:2002. The results of this study demonstrated fewer confirmed Salmonella positive plates from XLD and BG plating media when MKTTn was used, and more confirmed Salmonella positive when MSRV enrichment medium was used. This was in corroboration with those of several other studies which yielded more Salmonella positive plates when Rappaport-Vassiliadis rather than Tertrathionate selective enrichments were used [5] [11] [31] -[33] . Moreover, from the above observations, the combination of MSRV for selective enrichment and BG for selective plating media (MSRV/BG) may be the most appropriate in the detection and isolation of Salmonella from samples in the primary production of poultry.
Furthermore, in spite of the high sensitivity of XLD selective plating media over BG, the specificity of XLD was considerably low when compared to that of BG. This therefore affirms the superiority of MRSV/BG plating media over MSRV/XLD plating media for the detection of Salmonella species in poultry production systems. However, this is in disagreement with the traditional culture method for the isolation of Salmonella in feed stuff (ISO 6579, 2002), which uses XLD as the preferential selective plating media over the others (such as Brilliant Green agar, Salmonella Shigella agar, Hectoen Enteric agar etc.) for the isolation and detection of Salmonella. This contradiction may be as a result of the type of samples used in this study. The samples in this study included samples from the poultry environment (poultry feeds, poultry drinking water, poultry litter, poultry abattoir reins and dust from poultry house), from the poultry birds themselves (guts, cloacal swabs, poultry meat and eggs) and from the poultry personnel (poultry personnel stool and hand washings). Such a contradiction could have arisen as a result of the fact that different types of samples may be suitable for the detection of Salmonella using specific types of selective Plating media. Therefore, there is need for studies to be carried out to evaluate the performances of different selective plating media for the detection of Salmonella in different types of samples.
5. Conclusions
The results of this current study demonstrated that the type of selective enrichment media used (MSRV and MKTTn) had substantial effects on the sensitivity and specificity of the selective plating media (XLD and BG). MSRV selective enrichment medium was the most important, necessary for the investigation Salmonella in poultry production systems.
XLD plates demonstrated higher number of false positives than BG plates, while BG plates on the other hand demonstrated higher number of false negatives. In order to monitor food safety, it would be better to strike a bargain on a method that demonstrates fewer false positives than false negatives, of which BG selective plating media was the case with this current study. The combination of MSRV and BG revealed the highest sensitivity and specificity for the detection of Salmonella with fewer false positive plates. Therefore, based on the results of this study, the combination of MSRV/BG may render a better improvement for the detection of Salmonella, thereby minimising the cost of using two selective plating media in addition to the expensive and labour intensive biochemical and serological confirmations for the detection of Salmonella.
6. Limitation of the Study
MSRV is unable to detect non-motile Salmonella bacteria, which represent <1.0% of the isolates from poultry environment.
NOTES
*Corresponding author.