Influence of Silicate Structure on the Low Temperature Synthesis of Belite Cement from Different Siliceous Raw Materials ()
1. Introduction
Dicalcium silicate (belite, C2S) is the major components of Portland cement as same as tricalcium silicate (alite, C3S) and determines most of the adhesive properties, strength and durability of Portland cement. Belite hydrates much slower than alite. Whatever, belite shows about the same physical and mechanical properties as alite after complete hydration [1] . The synthesis of low-energy reactive belite cement is one of the most important challenges. Production of Portland cement consumes much of the fossil fuels (coke, petroleum and natural gas) [2] [3] , releasing about 5% of the total CO2 emission [2] [4] and the whole process needs high temperature for production (i.e. about 1450˚C). Recently, β-C2S was prepared from different siliceous raw materials such as silica fume, white sand, rice husk ash, silica, amorphous silica, oil well drilling mud and hydraulic dam sludge under various hydrothermal conditions with lime in presence in stabilizer such as BaCl2 or B2O3 followed by calcination of the product at 650˚C - 1000˚C [5] - [9] . The hydrothermal method used by previous researchers for synthesizing belite cement was modified to optimize the synthesis parameters [10] [11] . The hydrothermal treatment temperature was reduced to 100˚C and atmospheric pressure was used in presence of alkaline 0.6 M KOH solution using mixture of aluminosilicate waste. The aim of this work is to investigate the influence of silicate structure on the preparation of belite cement from different siliceous raw materials hydrothermally treated with lime and calcined at low temperatures.
2. Materials and Experimental Techniques
Lime was produced by calcination of limestone powder (purity > 99%) in an electrical muffle furnace at 950˚C for 3 hours. Lime was cooled to room temperature in desiccator, milled and stored in tightly closed plastic bag to avoid carbonation. White sand, metakaolin and dealuminated kaolin were used for preparation of belite. White sand and dealuminated kaolin were provided from Royal cement company, Minia
Egypt
and Egyptian Shaba company respectively. Metakaolin was prepared from high grade kaolin by calcination in muffle furnace at 750˚C for 3 hours. Distilled water and analytical grade barium chloride (BaCl2∙2H2O) were used without further purification. Mixtures of lime, BaCl2 with white sand, metakaolin or dealuminated [(Ca + Ba)/Si = 2] and distilled water (water/solid ratio of 5/1 by weight) placed in stainless steel capsule keeping the occupied volume equals 0.67 of total volume capacity. The capsule was tightly closed, shacked vigorously and hydrothermally treated at 180˚C for 5 hours in electric oven. The capsule was removed from oven and cooled to room temperature. The hydrothermally treated product was filtered, washed with distilled water, dried in an electric oven and calcined in an electric muffle furnace at 750˚C for 3 hours. The calcined product was cooled to room temperature in desiccator, milled and stored in tightly closed plastic bags. Mixtures of lime/BaCl2/white sand, lime/ BaCl2/metakaolin and lime/BaCl2/dealuminated kaolin were symbolized S, M and D respectively. X-ray diffraction (XRD) analysis were carried out by Philips x-ray diffractometer PW 1370,
Co.
with Ni filtered CuKα radiation (1.5406 Å). The Fourier transform infrared FTIR analysis was measured by spectrometer Perkin Elmer FTIR System Spectrum X in the range 400 - 4000 cm−1 with spectral resolution of 1 cm−1. Scanning electron microscopy SEM was investigated by Jeol-Dsm 5400 LG apparatus. The thermogravimetric TGA and differential thermogravimetric analyses DTG were carried out with the aid of Shimadzu Corporation thermo analyzer with DTG-60H detector with 10˚C/min heating rate from room temperature up to 1000˚C, under nitrogen atmosphere at 40 ml/min flow rate, the hold time at the appropriate temperature is zero.
3. Results and Discussion
Table 1 illustrates the chemical composition of limestone, sand, metakaolin, and dealuminated kaolin determined by XRF. The chemical composition results illustrate that; limestone composes of CaCO3, white sand composes of SiO2 while metakaolin was prepared from high grade kaolin. Dealuminated kaolin has higher (SiO2 + Al2O3 + Fe2O3) content compared to metakaolin and sand.
Figure 1 illustrates the XRD patterns of lime, sand, metakaolin and dealuminated kaolin. Lime composes of calcium oxide (CaO) in addition to small amount of portlandite (Ca(OH)2) that arises as a result of partial hydration of lime. White sand composes of quartz (SiO2) in addition to small amount of kaolinite (Al2O3.2SiO2.2H2O). The halo in the range 15 - 35 2θ, indicated that metakaolin has amorphous structure except that it contains residues of quartz. Dealuminated kaolin has amorphous structure except that it contains residues of quartz, kaolinite and wollastonite (CaO.SiO2). It is evident that studied siliceous materials have not only different content of SiO2 and chemical composition but also different phase composition and crystallinity of SiO2.
Figure 2 illustrates the FTIR spectra of lime, white sand, metakaolin and dealuminated kaolin. In case of lime (Figure 2a), the absorption band at 3640 cm−1 is due to OH-associated with portlandite (hydrated lime). The absorption band at 875 and 1441 are due to the v2 and v3 of carbonate (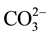 ) present as a partial carbonation of
) present as a partial carbonation of
![]()
Figure 1. XRD patterns of (a) metakaolin, (b) dealuminated kaolin, (c) lime and (d) white sand (K kaolinite, L lime, P portlandite, Q quartz, W wollas- tonite).
![]()
Figure 2. FTIR spectra of (a) lime, (b) white sand, (c) metakaolin and (d) dealuminated kaolin.
![]()
Table 1. The chemical composition of limestone, white sand, metakaolin, and dealuminated kaolin determined by XRF.
lime [12] [13] . The absorption band at 452 cm−1 is due to Ca-O stretching vibration [14] . In case of white sand, (Figure 2b), the absorption bands at 1084, 795 and 464 cm−1 are corresponding to Si-O-Si asymmetric stretching vibration, Si-O-Si symmetric stretching vibration and O-Si-O bending vibration respectively [15] - [17] . In case of metakaolin (Figure 2c), the band at 1632 and 3444 cm−1 corresponds to bending vibrations of free water molecules adsorbed to amorphous metakaolin surface. The broad band at 807 cm−1 corresponds to Si-O-Al vibrations and is characteristic of the degree of disorder in metakaolin structure [16] [17] . In case of dealuminated kaolin (Figure 2d), the bands at 3694 and 3620 cm−1 corresponds to stretching vibrations of hydroxyl groups which are sitting at the edges of the residual kaolin platelets as well as internal hydroxyl groups. The band at 917 cm−1 corresponds to the Al-O-H bending vibrations (hydroxyl groups sitting on the alumina faces). The band at 693 cm−1 correspond to Si-O-Si symmetric stretching vibration [17] . The band at 542 cm−1 correspond to Al4+-O-Si vibrations, where Al4+ is in octahedral coordination [16] .
Figure 3 illustrates the FTIR spectra of S, M and D mixtures hydrothermally treated at 180˚C for 5 hours. There is a significant change in the shape, position or intensity of the absorption bands at 1084, 795 and 464 cm−1 which are corresponding to Si-O-Si asymmetric stretching, Si-O-Si symmetric stretching and O-Si-O bending vibration respectively. The absorption band at 1084 cm−1 was shifted to lower wavenumber value at 1029 cm−1 whereas the intensity of absorption bands at 794 and 464 cm−1 were significantly reduced. The absorption band at 3449 cm−1 corresponds to vibration of combined water. Silica reacts with lime under hydrothermal conditions and forms dicalcium silicate hydrate. Accordingly, the degree of polymerization of silicate structure is lowered. Absorption bands at 3640, 452, 875 and 1441 cm−1 indicate the presence of unreacted lime, i.e. the hydrothermal treatment of the lime/silica mixture (Ca/Si = 2/1) at 180˚C for 5 hours does not drive the reaction to completion. The absorption band at 3640 cm−1 that corresponds to vibration of OH− groups associated with portlandite was significantly lowered in case of D mixture. This proves that dealuminated kaolin reacts with higher amount of lime compared to white sand and metakaolin because dealuminated kaolin enriched with amorphous silicate as confirmed by XRD results (Figure 1b).
Figure 4 illustrates the XRD patterns of S, M and D mixtures hydrothermally treated at 180˚C for 5 hours. The intensity of residual quartz SiO2 and portlandite Ca(OH)2 in hydrothermally treated mixtures decreases in the following descending order; sand ˃ metakaolin ˃ dealuminated kaolin. This indicates that the reactivity of silica rich material towards lime increases in the following ascending order; sand ˂ metakaolin ˂ dealuminated kaolin. Because sand composes of quartz crystals, metakaolin composes of amorphous metakaolin structure while dealuminated kaolin composes from enriched amorphous silicate. At the same time, S mixture show no sign for the existence of dicalcium silicate hydrate due to its amorphous nature. In contrast, M and D mixtures contain crystalline dicalcium silicate hydrate with lower degree of ordering due to its high content.
Figure 5 illustrates TGA/DTA thermograms of S, M and D mixtures hydrothermally treated at 180˚C for 5 hours. Result of thermal analysis was illustrated in Table 2. TGA/DTA thermograms illustrated the in situ sequence of thermal reactions and phase transformations that occur when hydrothermally treated mixtures were calcined up to 900˚C. Absorbed water was lost in the temperature range 80˚C - 130˚C [18] . Calcium aluminate and aluminosilicate hydrate dehydrates in the temperature range 300˚C - 350˚C [19] . Calcium aluminate and
![]()
Figure 3. FTIR spectra of (a) S, (b) M and (c) D mixtures hydrothermally treated at 180˚C for 5 hours.
![]()
Figure 4. XRD patterns of (a) S, (b) M and (c) D mixtures hydrothermally treated at 180˚C for 5 hours (K kaolinite, P portlandite, Q quartz, W wollastonite, D dicalcium silicate hydrate).
![]()
Figure 5. TGA and DTA thermograms of (a) S, (b) M and (c) D mixtures hydrothermally treated at 180˚C for 5 hours.
![]()
Table 2. Result of thermal analysis.
aluminosilicate hydrate only was observed in case of M mixture because metakaolin contains high amount of Al2O3 (42.13%) in contrast to white sand and dealuminated kaolin as illustrated by XRF analysis. Residual portlandite Ca(OH)2 dehydrates in the temperature range 450˚C - 550˚C [20] [21] . Dicalcium silicate crystallizes into β-C2S in the temperature range 590˚C - 760˚C. Finally, β-C2S transforms to α’-C2S in the temperature range 790˚C - 860˚C. The relative amount of residual portlandite in hydrothermally treated mixtures which detected from the weight loss in the temperature range 450˚C - 550˚C is in the following descending order; sand ˃ metakaolin ˃ dealuminated kaolin. This indicates that the reactivity of silica rich material towards lime increases in the same order as confirmed by FTIR and XRD results.
Figure 6 illustrates the SEM micrographs of S, M and D mixtures hydrothermally treated at 180˚C for 5 hours. Hydrothermally treated mixtures have different morphologies. S and M mixtures have small cube-like grains whereas D mixture enriched with large grains with rough surfaces. This observation agrees with the following predicted descending order of reactivity; dealuminated kaolin ˃ metakaolin ˃ white sand.
Figure 7 illustrates the FTIR spectra of S, M and D mixtures hydrothermally treated at 180˚C for 5 hours and calcined at 750˚C for 3 hours. The absorption band located at 1029 cm−1 was shifted to lower wavenumber value at 955 cm−1 and the absorption bands appearing at 876 and 520 cm−1 are characteristic to β-C2S [22] . The absorption bands at 1632 and 3433 cm−1 correspond to bending H-O-H vibration and O-H group stretching vibration of adsorbed free water molecules. The absorption bands at 3640 and 1429 cm−1 related to unreacted lime. The absorption band at 781 and 691 cm−1 related to Si-O-Si stretching vibration. Figure 8 illustrates XRD patterns of S, M and D mixtures hydrothermally treated at 180˚C for 5 hours and calcined at 750˚C for 3 hours. β-C2S formed due to dehydration of dicalcium silicate hydrate. There is no sign for the formation of γ-C2S. This proves that Ba2+ ions stabilized β-C2S and retards its transformation to γ-C2S because Ba2+ ions replace some of calcium atoms in the structure of β-C2S [23] . Figure 9 illustrates the SEM micrographs of S and D mixtures hydrothermally treated at 180˚C for 5 hours and calcined at 750˚C for 3 hours. It was observed that studied siliceous materials produce belite cement with different microstructure and phase composition as a result of its different SiO2, chemical composition and crystallinity of SiO2.
4. Conclusions
The main conclusions of this investigation are:
1) XRF, XRD and FTIR results of white sand, metakaolin and dealuminated kaolin prove that these siliceous materials have different proportions of SiO2 with different phase composition and crystallinity.
2) XRD, FTIR and SEM results of white sand, metakaolin and dealuminated kaolin hydrothermally treated with lime at 180˚C for 5 hours prove the formation of dicalcium silicate hydrate. Otherwise the hydrothermal treatment does not drive the reaction to completion. It is worth mentioning that dealuminated kaolin reacts with higher amount of lime compared to white sand and metakaolin and then form higher amount of dicalcium silicate hydrate.
3) TGA results give a picture of the thermal decomposition of white sand, metakaolin and dealuminated kaolin hydrothermally treated with lime at 180˚C for 5 hours. TGA results prove that β-C2S crystallizes in the temperature range 590˚C - 760˚C and transforms to α’-C2S in the temperature range 790˚C - 860˚C.
4) XRD, FTIR and SEM results of white sand, metakaolin and dealuminated kaolin hydrothermally treated with lime at 180˚C for 5 hours and calcined at 750˚C for 3 hours prove the formation of β-C2S. Ba2+ ions stabilized β-C2S and retarded its transformation to γ-C2S.
5) The silicate anion structure greatly affects the reactivity of siliceous raw materials towards lime under hy
![]()
Figure 6. SEM micrographs of (a) S, (b) M and (c) D mixtures hydrothermally treated at 180˚C for 5 hours.
![]()
Figure 7. FTIR spectra of (a) S, (b) M and (c) D mixtures hydrothermally treated at 180˚C for 5 hours and calcined at 750˚C for 3 hours.
![]()
Figure 8. XRD patterns of (a) S, (b) M and (c) D mixtures hydrothermally treated at 180˚C for 5 hours and calcined at 750˚C for 3 hours (P: portlandite, Q: quartz, β: belite, L: lime).
drothermal treatment. Dealuminated kaolin is more reactive compared to sand and metakaolin because of its amorphous silicate structure.
6) Based on current results, new studies in the future must be conducted to clarify the parameters which affect
![]()
Figure 9. SEM micrographs of (a) S and (b) D mixtures hydrot- hermally treated at 180˚C for 5 hours and calcined at 750˚C for 3 hours.
the reactivity of siliceous raw materials under hydrothermal treatment with lime in order to prepare low temperature belite cement.