1. Introduction
Now a day’s enzyme production is a growing field of biotechnology especially industrially important enzymes. In this regard, a wide spectrum of fungal species has been reported as a potential candidate to produce enzymes under optimal fermentation environment [1] [2] . Among them, Trichoderma is one of the most efficient candidates which has been extensively studied for the production of various industrially important enzymes mainly cellulases, exoglucanase, and β-glucosidase using various waste materials and their by-products such as wood, rice straw, banana waste, bagasse, corncobs, corn stover, wheat straw, rice husk, and bagasse [3] -[5] . Cellulose, hemicellulose and lignin are major components of plant cell wall and among them cellulose is the most abundant component of plant biomass, which comprises on average 35% to 50% of plant biomass [6] . Cellulose degrading enzymes system is a complex of three major types of enzymes that exhibit higher collective activity and degrade cellulose, a phenomenon known as synergism [1] . Currently, cellulases enzymes are being used in many industrial and biotechnological applications, especially in the fields of cotton processing, enzymatic hydrolysis of pretreated lignocelluloseic substrates, food industry, pulp and paper industry, bio-ethanol/bio-diesel, textile industry, animal feed stuff, and in research and development [3] -[9] .
By keeping in mind the extensive industrial applications, the present research work was especially focused with an aim to investigate the hyper-production of exoglucanase, and β-glucosidase using cheap and easily available agro-industrial residue corn stover as growth supporting substrate. To achieve the overall aim, various physio-chemical and nutritional variables were optimized using classical and completely randomized designs for induced production of exoglucanase, and β-glucosidase from the co-culture of Trichoderma viride and Ganoderma leucidum in solid state fermentation (SSF).
2. Materials and Methods
2.1. Chemicals and Substrate
All the chemicals used were of analytical laboratory grade. The agro-industrial waste, i.e. corn stover was obtained from a local fruit market in Gujrat, Pakistan. Corn stover was crushed into short pieces, dried and ground to fine powder size, and stored in plastic jars.
2.2. Fungal Cultures and Inoculum Development
To develop homogeneous inoculums suspension, spores of the both cultures were cultivated at 30˚C for 5 - 7 days using 250 mL capacity Erlenmeyer flask containing 30 mL of Potato Dextrose broth. This was then incubated under stationary conditions for the development of the fungal spore suspension.
2.3. Pre-Treatment of Corn Stover
10 g corn stover was pre-treated with 2% HCl in an Erlenmeyer flask at room temperature for 2 h then autoclaved at 121˚C and 15 lb/in2 pressure for 15 min. The slurry of corn stover was filtered through Watman No 1 filter paper; both the filtrates and the residues were saved and used for the production of exoglucanase, and β- glucosidase enzymes and further analysis.
2.4. Fermentation Strategy
10 g of pre-treated corn stover was moistened with nutrient salt media in an Erlenmeyer flask for enzyme production. The initial pH of the fermentation medium was adjusted to 5 before sterilization, inoculated with 5 mL of freshly prepared fungal co-culture spore suspension, and incubated at 30˚C in an incubator for stipulated fermentation time period.
2.5. Optimization of Physicochemical and Nutritional Variables
2.5.1. Effect of Time Period
To optimize the fermentation time period, each flask containing pre-treated corn stover was sterilized, inoculated, and fermented at 30˚C for ten days time period, in a still culture temperature controlled incubator. Flasks were harvested in duplicate after every 24 h of fermentation time and analyzed for exoglucanase, and β-glucosidase activity.
2.5.2. Effect of Different Solvents
The effectiveness of extraction is necessary towards the recovery of enzyme from the fermented biomass. Thus different solvents i.e., distilled water, tab water, tween-80, citrate and phosphate buffer of pH 4.8 were investigated for the recovery of exoglucanase, and β-glucosidase.
2.5.3. Effect of pH and Temperature
Fermentation media was adjusted to varying pH levels (3 - 7) before inoculation with 5 mL of fresh spore suspension and allowed to ferment for 6 days fermentation time period. To obtain the maximum enzyme production, duplicate flasks containing pre-treated corn stover were adjusted to pH 6, inoculated, and subjected to fermentation at varying temperatures ranging from 25˚ to 45˚C.
2.5.4. Effect of Substrate Level
To investigate the optimum substrate level for the production of exoglucanase, and β-glucosidase, varying levels of pre-treated substrate (5, 10, 15, 20, and 25 g/100 mL) were used. Duplicate flasks inoculated with fungal cocultures were subjected to fermentation for the optimum time period (6 days) at pH 6 and 40˚C.
2.5.5. Effect of Various Carbon and Nitrogen Sources
The effect of various carbon and nitrogen sources as an additional nutrient supplements were examined for their effect on exoglucanase, and β-glucosidase production. The carbon sources used were: glucose, fructose, sucrose, maltose, and molasses while the additional nitrogen sources were urea, yeast extract, beef extract, peptone, and ammonium sulfate.
2.5.6. Effect of Inoculum Size
To determine the optimum inoculum level that would give the best enzyme production, the duplicate flasks were inoculated with varying volumes (2 to 10 mL) of freshly prepared co-cultures inoculums and then processed for 6 days at optimum pH and temperature.
2.6. Extraction of Crude Enzyme Complex
The crude enzyme complex was extracted from the fermented corn stover biomass via the addition of a citrate buffer, 0.05 M of pH 4.8 in a 1:10 (w/v) ratio, and then the flasks were shaken at 120 rpm for 30 min [1] . The contents were filtered through Wattman No 1 filter paper and washed twice with the same buffer. The filtrates were centrifuged at 10,000 × g for 10 min, and the collected supernatants were used as a crude enzyme extract to determine the activity.
2.7. Enzyme Activity Assays
Exoglucanase was assayed according to the method of Deshpande et al. [10] ; 1% salicin was used as a reaction substrate with DNS as a coupling reagent. The β-glucosidase activity was determined by the method of Gielkens [11] .
3. Results and Discussion
3.1. Optimization of Fermentation Time Period
The enzyme activity profile from the fermentation time screening trials revealed that the maximal activities of exoglucanase, and β-glucosidase were 115 ± 3.2 U/mL and 97 ± 0.3 U/mL recoded after the 5th day of inoculation with fresh spores of T. viride and G. leucidum (Figure 1). It was also observed that in the later stage (after optimal time period) the death phase from the microbial life spam may cause the inactivation/inhibition of the enzymes produced. In an earlier study, Quiroz-Castañeda et al. [7] has achieved maximum activity of the cellulases after 8 days of inoculation using wheat straw as a growth substrate. In present study, co-culture of T. viride and G. leucidum produced higher titters of exoglucanase, and β-glucosidase in comparison to previously studied different fungi which produced maximum enzymes after 6 - 8 days of fermentation [7] .
3.2. Effect of Different Solvents
The effectiveness of extraction is necessary towards the recovery of enzyme from the fermented biomass. Therefore different solvents were investigated for the recovery of exoglucanase, and β-glucosidase. The activity
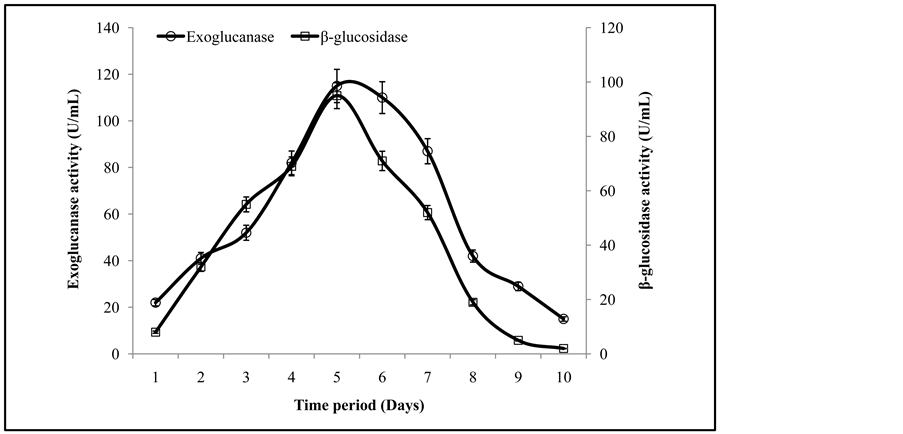
Figure 1. Activities of exoglucanase, and β-glucosidase under different incubation time period.
analysis of culture extracted showed its maximum activity of exoglucanase (196 ± 2.1 U/mL), and β-glucosidase (135 ± 1.5 U/mL) (Figure 2) when was recovered with a 0.05 M citrate buffer of pH 4.8 while minimum enzyme activities of exoglucanase, and β-glucosidase were obtained with a tab water. While in contradiction to the present investigations, Chandra et al. [12] reported a single wash with 20 mL distilled water gave maximum yield (10.87 Ug−1) among different solvents used for the recovery of enzyme.
3.3. Optimization of pH & Temperature
Figure 3 illustrates that the initial enzyme activity increased with the increase of pH and the peak activity was at pH 6, while further increase in pH showed decreasing trend in enzyme activity. It has been reported that the optimal pH for fungal cellulases varies from species to species. Similar results are also reported by Pushalkar et al. [13] , who found that β-glucosidase was more active on the substrate between pH 4.0 and 5.5. Enzyme activity recorded at different temperatures revealed that a co-culture of T. viride and G. leucidum yielded maximum production of exoglucanase and β-glucosidase at 35˚C (Figure 4). The same phenomenon of incubation temperature has also been reported by Omojasola and Jilani [14] . Similar to our findings, an incubation temperature of 30˚C was optimal for the production of cellulase (CMCase) from Trichoderma harzianum [1] .
3.4. Optimization of Substrate Concentration
The maximum exoglucanase (330 ± 3.3 U/mL) and β-glucosidase (190 ± 2.2 U/mL) activities were obtained from the culture fermented with 15 g substrate concentration (Figure 5). Earlier it has been reported that substrate concentration is a dynamic influencing factor that affects the product yield and the initial hydrolysis rate of cellulose [1] . A high substrate concentration can cause substrate inhibition, which substantially cause the reduction in enzyme formation [5] .
3.5. Effect of Carbon and Nitrogen Sources
To investigate the effect of carbon and nitrogen sources, Completely Randomized Design (CRD) was used. Glucose, fructose, sucrose, maltose and molasses (1%) as carbon sources along with (0.2%) nitrogen supplements like urea, yeast extract, beef extract, peptone and ammonium sulphate were used in different interaction for highest production of exoglucanase and β-glucosidase to study their stimulatory/inhibitory effects under optimized conditions. It was important to note that by the addition of carbon and nitrogen sources, the production pattern of enzymes changed; the combination of glucose and ammonium sulphate proved best and gave maximal production of exoglucanase (399 ± 4.1 U/mL) (Table 1), and β-glucosidase (230 ± 3.6 U/mL) (Table 2). The enzyme profiles varied with different carbon and nitrogen source combinations. The source and concentration of
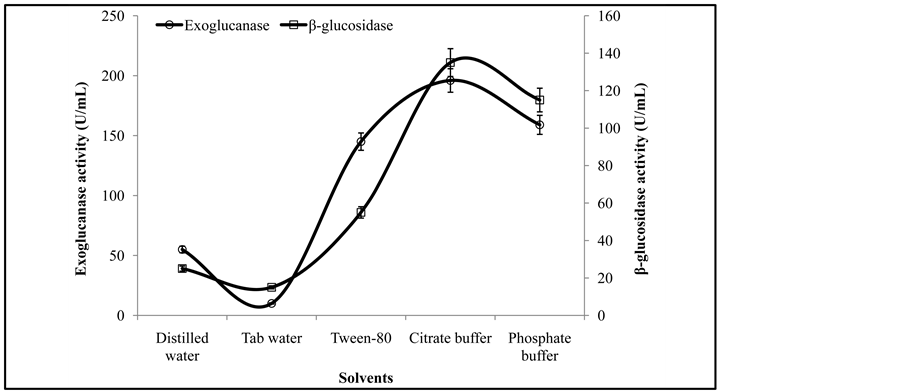
Figure 2. Activities of exoglucanase, and β-glucosidase under various solvents.
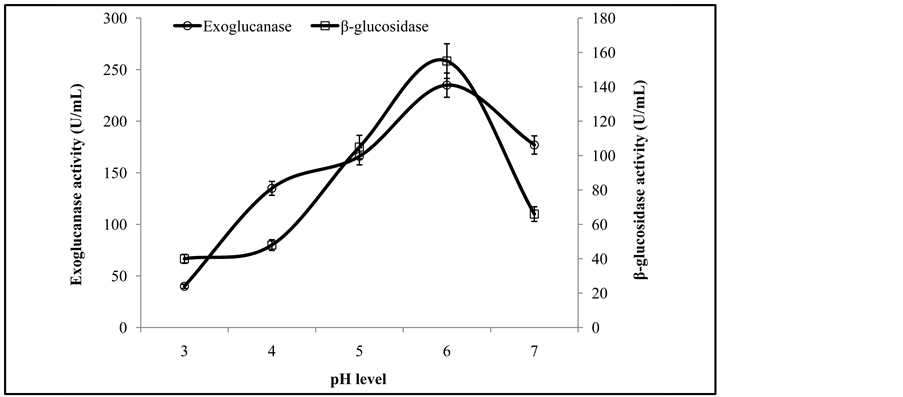
Figure 3. Activities of exoglucanase, and β-glucosidase at different pH levels.
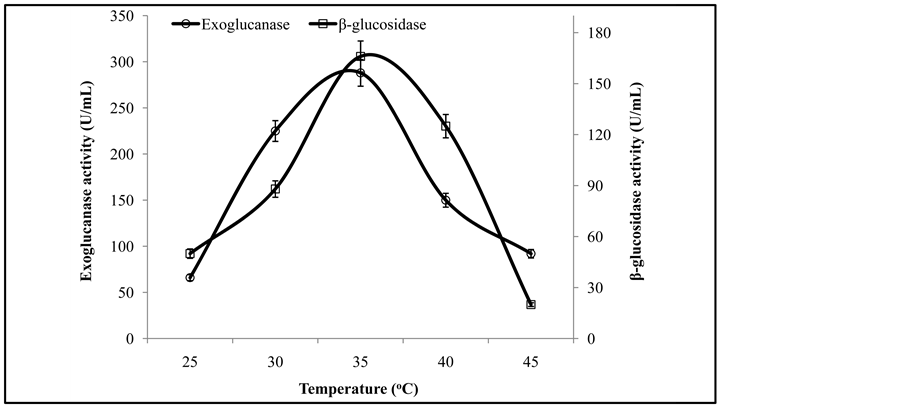
Figure 4. Activities of exoglucanase, and β-glucosidase under varying temperatures.
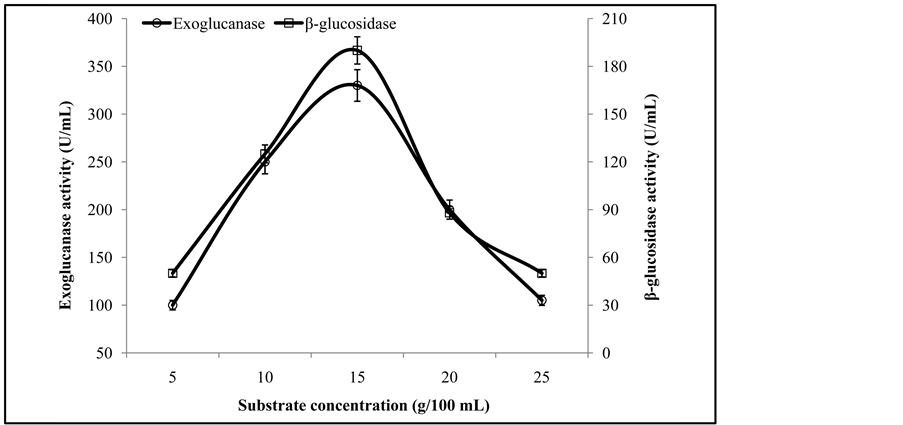
Figure 5. Activities of exoglucanase, and β-glucosidase on different substrate levels.

Table 1. Activities of Exoglucanase produced from the co-culture of T. viride and G.lucidum in solid state fermentation with different carbon and nitrogen sources.

Table 2.Activities of β-glucosidase produced from the co-culture of T. viride and G. lucidum in solid state fermentation with different carbon and nitrogen sources.
carbon and nitrogen are the powerful factors regulating the synthesis of enzymes by white rot fungi (WRF) [4] [15] .
3.6. Optimization of Inoculum Size
Optimum spore density is important for SSF process. The maximal exoglucanase and β-glucosidase activities of 485 ± 6.5 U/mL and 255 ± 3.3 U/mL respectively, were noted in the experimental flasks receiving 6 mL inoculum density of co-culture (T. viride and G. leucidum) (Figure 6). Lower inoculum sizes shortened the microbial
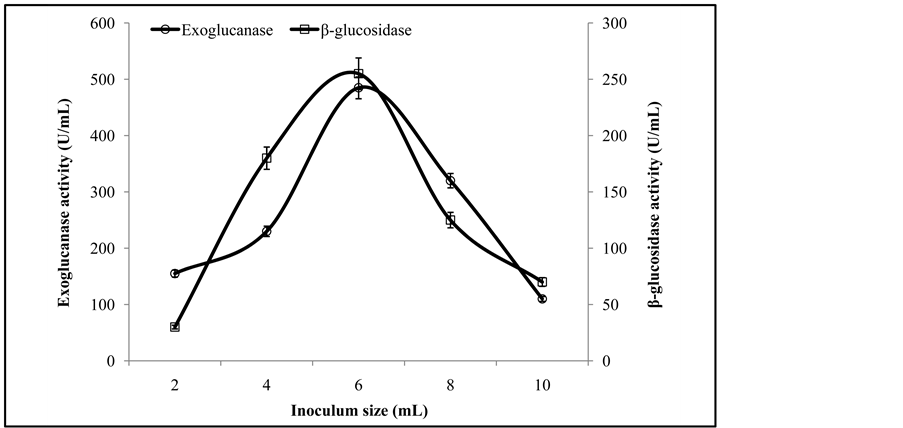
Figure 6. Activities of exoglucanase, and β-glucosidase on varying sizes of fungal inoculums.
lag phase stage, whereas inoculum size beyond the optimum value increased the moisture factor that caused lower levels of enzyme formation due to the overcrowding of fungal spores [3] -[5] . In an earlier study Omojasola and Jilani [14] reported the maximum cellulase activity with an inoculum size of 8%.
4. Conclusion
The results of this study indicate notable cellulases production potential of T. viride and G. leucidum from corn stover. In conclusion, attempt was made to find the optimum fermentation conditions for successful cultivation of microbial co-cultures. However, the suitability of the enzymes for biotechnological applications can be investigated through kinetic characterization of the purified enzymes as thermo-stability is a desired characteristic of an enzyme for its possible use in industry.
Acknowledgements
On providing financial support and laboratory facilities authors are grateful to the Department of Biochemistry NSMC University of Gujrat, Pakistan.
NOTES
*Corresponding author.