Study of Physicochemical Parameters of Rainwater: A Case Study of Karachi, Pakistan ()
1. Introduction
Rain acts as a powerful mechanism to remove pollutants from the atmosphere. Precipitation chemistry is the result of a series of in-cloud and below-cloud atmospheric chemical reactions and a complex interaction between microphysical processes and cloud dynamics [1] . Rainwater composition is important in evaluating the role of transport of soluble material and the contribution of different sources of atmospheric pollutants.
Aerosols and gases released into the atmosphere can be transported over long distance from their sources, and can be removed by dry or wet deposition [2] [3] . Every day, large quantities of anthropogenic and natural material are dumped into the atmosphere and majority of this material returns to the ground. Man is primarily responsible for the enrichment of many trace elements in the atmosphere caused by the combustion of fossil fuels, including such additives as lead in gasoline, processing of crustal materials for manufacturing cements, roasting of ores for refining metals, and burning of waste materials [4] . Many industrial processes produce large quantities of oxides of sulfur and nitrogen is being emitted to the atmosphere, and these gases are being converted into strong acids, which lead to many areas experiencing precipitation of very low pH values [5] -[7] . Composition of rainwater varies from site to site and it is difficult to control as it is influenced by both natural and anthropogenic sources. If the source is influenced by man made activities more, it will contribute to acidic gases like NOx and SOx due to which rainwater becomes acidic and basic gases like NH3 [8] [9] . Environmental adverse effects of acid rain include changes in the leaching rates of nutrients from plant foliage and soil nutrients, acidification of lakes and rivers, effects on metabolism of organisms and corrosion of structures [10] [11] . In addition to that, acid rain is also responsible for reduction of visibility and deterioration of historical structures especially by damaging the details on historic places or sculptures [5] [12] . Precipitation composition thus is an indispensable measurement and helps to understand the comparative importance of the different sources of these materials.
Because of this concern, precipitation chemistry in both rural and urban areas has been the subject of intense research in the last two decades [13] -[16] . Currently, there are more than 1000 stations conducting precipitation chemistry measurements around the world. All these working stations may be categorized into global, regional and local networks. Global networks comprising the BAPMON (Background Air Pollution Monitoring Network) and GPCP (Global Precipitation Chemistry Project) sites collect precipitation at remote areas and provide worldwide information on the background concentration of air pollutants and long range transport of trace substances in the atmosphere. A large number of local networks are operated in several countries, which address specific scientific questions, abatement strategies and pollution issues. In various countries like Europe, North America regional networks are functioning and documenting spatial and long term trends in deposition of acidic materials [17] .
Karachi is one of the largest mega cities of the world and most populous city of Pakistan, serving as the primary center of finance, commerce and industry. It is spread over approximately 3530 km2 (1362 sq·mi) in area [18] . The city population is growing at an alarming rate and results in the rapid increase of vehicle number (>1.3 million) and energy consumption due to which air pollution situation has become severe in the city. The principal thrust of this study is to use the rainwater as a tool to examine the extent of air pollution and to compare the major physicochemical parameters to the WHO water standard guidelines. In the same series of research work a comprehensive article regarding the physicochemical assessment of rainwater of 2007 is recently published [19] . In the year 2009, 2010, 2011 and 2012 the precipitation events were very rare so no spatial sampling and analysis is done. Therefore, it is a second systematic report on the study of rainwater analysis of Karachi, Pakistan.
2. Materials and Methods
Rainwater samples were collected from different areas of Karachi city in the monsoon season of 2013, in clean polypropylene bowls. The bowls are placed 2 m above the ground level to avoid contamination from the ground. The rainwater samples were then transferred to the polypropylene bottles that were pre-rinsed with deionized water and properly caped to prevent the oxidation of the constituents. A total of fifty four samples from eighteen towns of Karachi were collected (Figure 1) and their pH, EC and DO were measured immediately. Each sample was filtered and divided into two portions. One part was stored at 4˚C for anion analysis and other portion was acidified with HNO3 (BDH) for cation analysis. The major cations were measured by Perkin-Elmer atomic absorption spectrophotometer (AAnalyst 700). Anions were analyzed by ion chromatography (Metrohm 761 Compact IC with suppressed module, equipped with an anion-separator column (Dual 2). pH, EC, DO and NH4
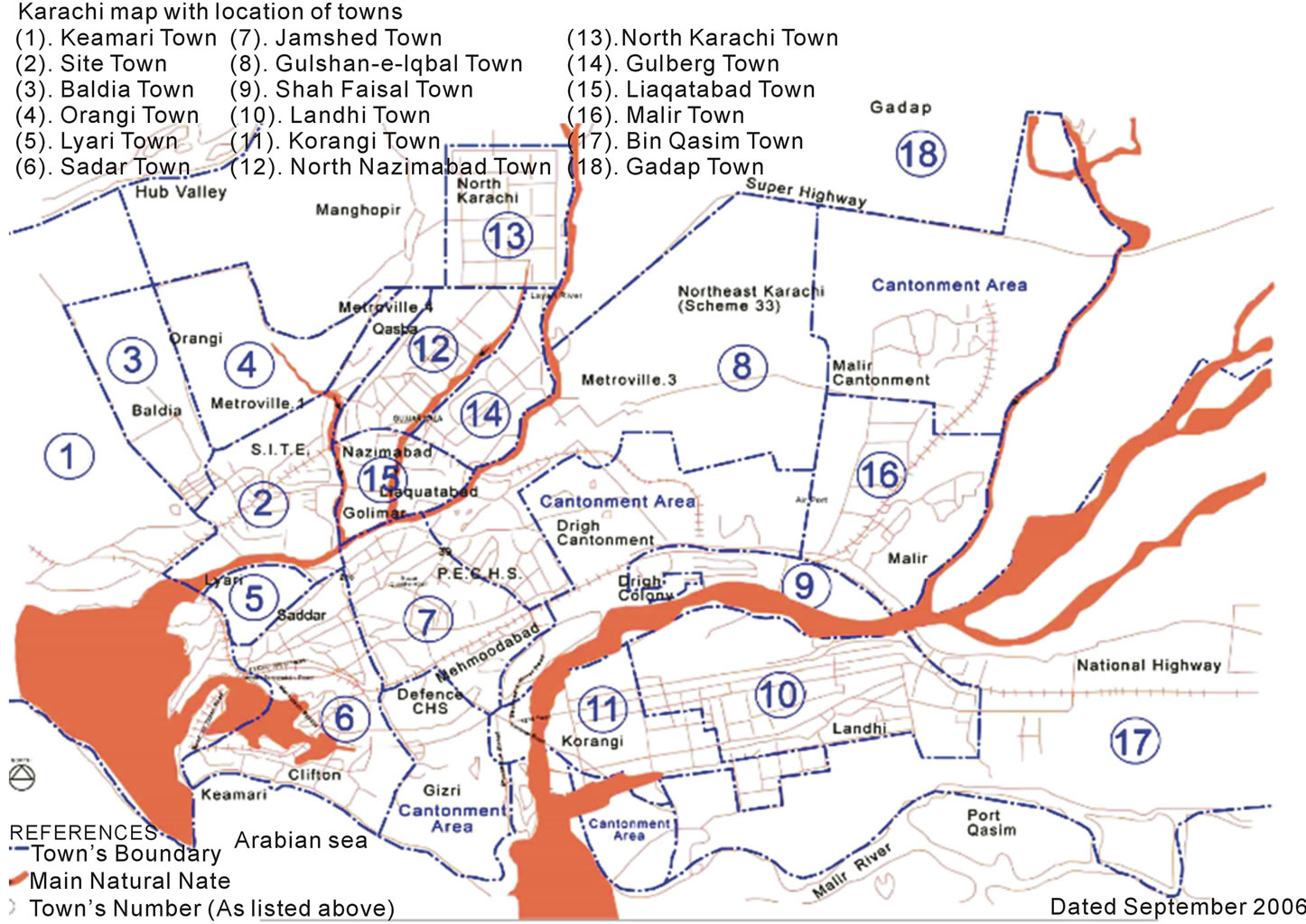
Figure 1. Geographical map of Karachi showing the location of sampling sites.
was analyzed by multiparameter ion analyzer (HANNA Instrument).
3. Results and Discussion
Atmosphere of Karachi is greatly influenced by the rapid urbanization which results in increased levels of atmospheric pollutants. Rainwater is an essential part of hydrological cycle that plays an important role in the global cycling of soluble chemicals in water. It serves as a cleanser of the atmosphere, washing out pollutants from air and introducing them into surface water and soil where it may have adverse effects on natural ecosystem. This study was aid to understand the effect of natural and anthropogenic effects on the quality of rainwater. In order to estimate the effect of pollution in air we analyzed rainwater samples collected from eighteen towns of Karachi, Pakistan, and results are summarized in Tables 1 and 2.
3.1. Physical Parameters of Rainwater
The results of the field measurements (pH and Total Dissolved Solids) and those obtained after analysis of the rainwater samples in the laboratory are shown in Figure 2.
3.1.1. pH
The acidity of rainwater depends on the concentration of anionic as well as cationic species. Acidic pH reveals the presence of strong acids while neutral or alkaline pH indicates neutralization of acids by carbonates, mineral dust or by ammonium. This may be due to the reaction of sulphuric and nitric acid absorbed in the aerosols with alkaline carbonates in the particulate matter [20] -[22] . pH is a useful tool for measuring the acidity of rainwater. The results demonstrated that no acid rain event is observed in Karachi as the recorded pH values of rainwater samples ranged from 6.2 - 7.9. The minimum pH was found in Saddar and Binqasim while highest concentration was found in Korangi industrial area.

Table 1. Physical parameters (pH, EC, TDS, DO & hardness ± standard deviation) of rainwater samples at different sites of Karachi.
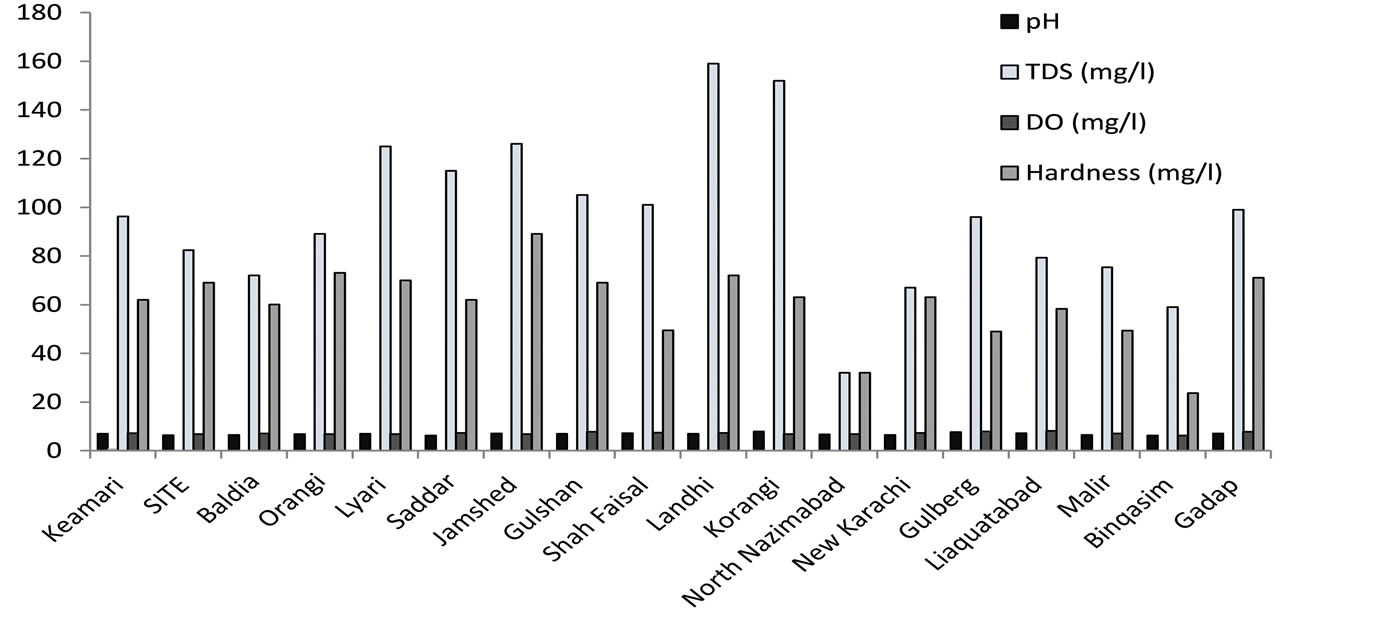
Figure 2. The physical parameters of rainwater at several places in Karachi, Pakistan.
3.1.2. Conductivity
The electrical conductivity is referred as key factor for determining the purity of water that depends on nature and concentration of ionized substances (chloride, nitrate, sulphate, and phosphate anions or sodium, magnesium, calcium, iron, and aluminium cations) in the water at water temperature. The conductivity in Karachi rainwater samples ranged between 0.02 - 0.21 m·S/cm. The minimum conductivity value was found in New Karachi where as maximum value is found in Korangi sample.
It is clear from the value of electrical conductivity of the samples that the rainwater of Karachi is free from considerable contamination of cations and anions from the atmosphere and it is pure water. Pure water also undergoes dissociation in which, H3O+, OH− ions presents in small quantity that shows little conductivity. The Table 2. Chemical composition of rainwater samples at different sites of Karachi in mg/l ± standard deviation.
conductivity values obtained from rainwater analysis meets the WHO (World Health Organization) guideline values of conductivity of safe drinking water [23] .
3.1.3. Total Dissolved Solids
Total dissolved solids (TDS) are the solids present in water in the dissolved form. TDS is very significant parameter describing the chemical constituents of the water and can be considered as general of edaphically relation that contributes to productivity within the water body [24] . Sulphurous and carbonic acids are present in rainwater but they have no effect on its hardness. It is clear from the TDS of rainwater that more is the TDS value more will be the suspended and dust particles in it. TDS of rainwater also depends upon quantity of rain fall. The TDS values also show that the rainwater can be described as fresh. Based on the Australian and UNESCO standard for livestock which stated that the TDS values between 0 to 2900 mg/l are suitable for all animals [25] . The values obtained in rainwater samples indicate that it is suitable for livestock farming since the TDS ranged from 32 to 159 mg/l. The minimum concentration found in North Nazimabad where as maximum concentration was found in Landhi sample.
3.1.4. Dissolved Oxygen
Assessment of dissolved oxygen (DO) is a major characteristic in all pollution related ecological studies [26] . The value of dissolved oxygen in samples ranged from 6.3 - 8.2 mg/l. The lowest value was observed in Binqasim sample and the highest value was observed in Liaquatabad sample.
3.1.5. Hardness
The total hardness of the rainwater varied from 23 to 89 mg/l. The lowest value is found in Binqasim rainwater sample while the highest value is found in Jamshed town sample. In only one sample hardness exceed the WHO (World Health Organization) standard value [23] . The low concentration of hardness is due to low concentration of calcium and magnesium ions in the rainwater. Hence, it can be said that rainwater is not hard and it can be used for washing purpose.
3.2. Major Cations and Anions
Rainwater is a mixed electrolyte that contains varying amounts of major and minor ions. Sodium, potassium, magnesium, calcium, chloride, bicarbonate, and sulphate ions are major constituents, together with ammonia, nitrate, nitrite and other nitrogenous compounds.
There is a considerable range in the amounts of the ions present in rainwater of Karachi (Figures 3 and 4).
The main source of dissolved materials such as Na+ and Cl−, in the rainwater is sea salt. When waves split, fine droplets of seawater are injected into the atmosphere. The water evaporates, leaving a solid aerosol particle, which is transported by winds until it is dissolved by rain. This process is responsible for the high concentrations of Na+ and Cl− in rainwater. The concentrations of Na+ ranged in between 20.37 to 192.53 mg/l and Cl− concentrations ranged from 25.32 to 149.0 mg/l. Ca2+, Mg2+ and K+ ions in rainwater are presumably come both from oceanic salts and dust in the atmosphere from land surface. The concentrations of Ca2+ varied from 28.92 to 119.58 mg/l; while that of Mg2+ and K+ varied from 25.22 to 87.54 mg/l and 4.37 to 12.98 mg/l, respectively.
The concentration of Mg2+ in Lyari, Gulshan, Shah Faisal, Landhi, Malir and Gadap samples is comparatively higher from the WHO standard values of fresh water i.e. 50 mg/l.  is derived from plants, agriculture animal waste and fertilizers, and automobile exhausts. It is one of the major nitrogenous contributions to the chemical composition of precipitation. The HNO3 (gaseous) which results from the oxidation of NOx is water soluble, meaning it is washed away by precipitation, constituting one of the most important sources of
is derived from plants, agriculture animal waste and fertilizers, and automobile exhausts. It is one of the major nitrogenous contributions to the chemical composition of precipitation. The HNO3 (gaseous) which results from the oxidation of NOx is water soluble, meaning it is washed away by precipitation, constituting one of the most important sources of  in rainwater [27] . In the case of nitrate, it is important to consider the variations in the efficiency of conversion from NO2 to gaseous HNO3. In summer, this conversion occurs rapidly, while in the winter it is slower [28] [29] . The concentration of nitrate in rainwater samples of Karachi is ranged between 13.72 - 83.99 mg/l. Minimum concentration was found in Shah Faisal colony sample while maximum concentration was found in Korangisample. The rainwater of North Nazimabad, Korangi, Gulshan, Saddar, Lyari, SITE and Keameri are more ex-
in rainwater [27] . In the case of nitrate, it is important to consider the variations in the efficiency of conversion from NO2 to gaseous HNO3. In summer, this conversion occurs rapidly, while in the winter it is slower [28] [29] . The concentration of nitrate in rainwater samples of Karachi is ranged between 13.72 - 83.99 mg/l. Minimum concentration was found in Shah Faisal colony sample while maximum concentration was found in Korangisample. The rainwater of North Nazimabad, Korangi, Gulshan, Saddar, Lyari, SITE and Keameri are more ex-

Figure 3. The concentration of cations in rainwater at several places in Karachi, Pakistan.

Figure 4. The concentration of anions in rainwater at several places in Karachi, Pakistan.
posed to automobile exhaust and they are found contaminated with more  as compare to other areas samples.
as compare to other areas samples.
The second contribution to nitrogenous rainwater composition is of ammonium. Its presence in precipitations results in the condensation of aerosols containing ammonium and of the incorporation of gaseous ammonia in cloud droplets. Major sources of ammonia are known to be natural or fertilized soils, excrements of human and animals, and wood burnings. Also, in the atmosphere ammonium that mainly associated with farming and biomass burning mainly combines with sulphate and nitrate to form ammonium sulphate and ammonium nitrate, respectively [30] -[32] . The concentrations of  ranged from 32.39 to 198.52 mg/l. The minimum concentration of ammonium is found in Keamari rainwater sample i.e. 32.39 mg/l where as maximum concentration of ammonium is found in New Karachi samples i.e. 198.52 mg/l.
ranged from 32.39 to 198.52 mg/l. The minimum concentration of ammonium is found in Keamari rainwater sample i.e. 32.39 mg/l where as maximum concentration of ammonium is found in New Karachi samples i.e. 198.52 mg/l.
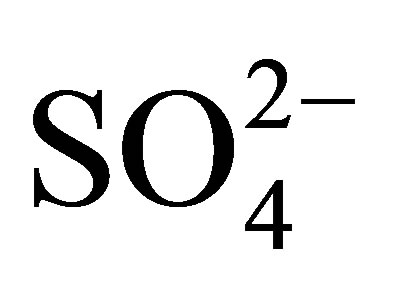 is formed by SO2 gas emission from automobile exhaust. It reacts with water droplets and accounts for the source of sulphate ions in rainwater. In collected samples the
is formed by SO2 gas emission from automobile exhaust. It reacts with water droplets and accounts for the source of sulphate ions in rainwater. In collected samples the  ranged from 20.45 to 98.36 mg/l. The highest concentration is found in Korangi and lowest concentration is found in Malir.
ranged from 20.45 to 98.36 mg/l. The highest concentration is found in Korangi and lowest concentration is found in Malir. 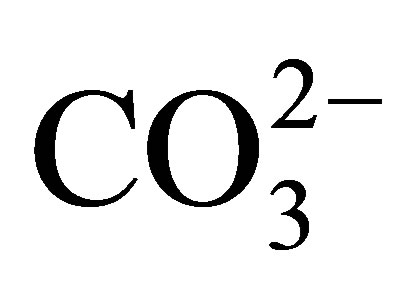 and
and  in rainwater is formed by the dissociation of carbonic acid formed by the reaction of carbon dioxide gas with water [33] . The concentrations of
in rainwater is formed by the dissociation of carbonic acid formed by the reaction of carbon dioxide gas with water [33] . The concentrations of  in Karachi rainwater varied from lowest value of 19.85 mg/l in Binqasim to highest value of 73.05 mg/l in New Karachi samples.
in Karachi rainwater varied from lowest value of 19.85 mg/l in Binqasim to highest value of 73.05 mg/l in New Karachi samples.
In general, the results indicate that the concentrations of the constituent major cations and anions are low and this is typical for most rainwater resources in industrialized urban areas [34] . Moreover, the concentration of major cations and anions of the rainwater conform to the standard for safe drinking water.
4. Conclusions
The chemical contents of rainwater form a useful parameter to disclose the characteristics of rain in question. Therefore, it is concluded from the analysis that the rainwater of Karachi city is not polluted significantly.
Although, further studies are needed in order to complete this data with aspects such as organic deposition or dry deposition in order to create a complete database that permits evaluating modelling exercises and improving knowledge about future environmental and human health impacts.
Acknowledgements
The authors are extremely thankful to PCSIR Laboratories Complex Karachi-75280, Pakistan for providing instrumental facilities for the analysis of rainwater samples .
NOTES
*Corresponding author.