Apelin is synthesized as pre-proapelin that is isolated in many tissues. Multiple isoforms are identified as apelin-36, apelin-17, apelin-13 that are considered to exist in vivo [2]. Apelin-36 is assumed to be the physiologically active one. The biological activity of apelin is focused in the myocardium, endocardium endothelium in the large vessels as well as in the small arteries and veins [6]. Sources stand for the idea that apelin plays an important role in the endothelial cell proliferation (like malignant neoplasms or diabetic retinopathy) [9]. Apelin has a hypotensive effect when applied intravenously in rats as it lowers systolic and diastolic blood pressure by 10 mmHg [10]. Applying NO-synthetase inhibitor blocks this effect which assumes a NO-mediated mechanism-endotheliummediated vasodilatation [11]. Although apelin could lead to vasoconstriction in case of endothelium damage that the smooth muscle cells are the affected cells and they also have APJ-receptors [12].
Considering the expression of apelin and the APJ-receptor in different areas of the central nervous system, especially in some hypothalamic nuclei, it is proposed that apelinergic system could be involved in the hypothalamic-pituitary axis and the water balance. It was demonstrated that 30 minutes after injecting apelin, the levels of ACTH and serum cortisol raise, and prolactine, LH and FSH drop [13]. A correlation was established with insulin and obesity as plasma apelin levels are much higher in obese mice with hyperinsulinaemia and are much lower in insulin-dependent rats with low fasting insulin levels. The correlation with insulin gets much more interesting after expression of APJ-receptor was established in the pancreas and inhibition of the glucosedependent insulin secretion by apelin was demonstrated through direct action on mice beta-cells [14]. Significantly higher plasma apelin levels were found in obese than in normal-weight men [1]. A positive correlation of serum apelin levels and body mass index was found [15]. Also higher apelin levels were measured in diabetes mellitus and impaired carbohydrate tolerance [16]. When applied intraperitoneally to normal and obese mice for a period of 14 days, apelin was found to reduce fat tissue without changing diet; to reduce insulin levels, leptin and triglycerides and to raise level of adiponectine [17]. Details about the physiologic role of apelin and its interaction with the elements of metabolic syndrome (MS) are to be figured.
The role of late-onset hypogonadism (LOH) wasn’t revealed so far and it is also considered as a part of MS. Most of the components of the metabolic syndrome (obesity, hypertension, dislipidaemia, glucose metabolism abnormalities and insulin resistance) are also found in men with hypogonadism. Epidemiologic studies have established connection between obesity and low serum testosterone levels in healthy men [18]. 20% to 64% of men with obesity have low total (TT) or free (FT) testosterone levels [19]. Kupelian et al. have established that low TT and low levels of sex-hormone binding globulin are risk factors for metabolic syndrome even in symptomless androgenic deficit [20]. This confirms the data of Muller et al., that endogenous levels of sex hormones are low in MS [21]. Other authors find inverse correlation of number of components of MS and levels of TT [22]. It is assumed that the more parameters are abnormal, and lower levels of testosterone would be measured [23]. Large longitudinal studies confirm that frequency of MS is higher in elderly men and hypogonadism is a relevant factor [20,24]. The relation of hypogonadism and MS is stable when different definitions of MS are applied in prospective monitoring of patients for more than 10 years [25]. We did not find data about the levels of apelin in men with LOH.
The aim of this study is to determine the levels of apelin in men with MS with low or normal testosterone levels and to monitor apelin levels in testosterone replacement therapy.
2. Patients and Methods
2.1. Metabolic Syndrome
In this study are included consecutive patients of the Clinic Endocrinology of Alexandrovska University Hospital in Sofia for the period 03.2010-02.2011. All men signed informed consent before entering the study which was approved by the local ethics committee. At first they are clinically analyzed. Data about medical history, physical examination, height, weight, waist and hip circumference, blood pressure are gathered. Standard metabolic markers, as well as the hormonal status of the gonadal axis are measured in the central laboratory of the hospital, which is the reference center for the country. We calculate:
• body mass index (BMI) = weight/height2 (kg/m2);
• homeostatic index of insulin resistance HOMA = fasting blood glucose*fasting insulin/22.4;
• creatinine clearance by the formula of Cockroft-Gault Ccr = (140 − age)*weight/0.814*serum creatinine;
• FT and bioavailable (BT) testosterone by the Vermeulen formula (internet calculator).
Defining the body composition—fat and fat-free body mass, body water we use bioimpedance analyzer Tanita TBF-215 (Tanita, Japan).
According the criteria of International Diabetes Federation 2005 [26] abdominal obesity plus two of the other criteria are needed for the diagnosis of the metabolic syndrome. We analyzed:
• abdominal obesity (waist circumference ≥ 94 cm or BMI > 30 kg/m2);
• carbohydrate metabolism − fasting plasma glucose ≥ 5.6 mmol/l or previously diagnosed type 2 diabetes (T2DM);
• blood pressure (BP) − systolic (SBP) ≥ 130 or diastolic (DBP) ≥ 85 mmHg, or already on medication for arterial hypertension;
• level of high density lipoproteins (HDL) < 1.03 mmol/l;
• level of triglycerides (TG) ≥ 1.7 mmol/l.
2.2. Late-Onset Hypogonadism—LOH
By definition low serum testosterone and clinical signs of testosterone deficiency are needed to set this diagnosis.
Total testosterone (TT) is measured twice in different days in the morning on fasting state. Of this two results mean arithmetic is calculated and used as TT-level for the purposes of the study. We accept the level 10.4 nmol/l as a cut-off level for the testosterone deficiency as used by other authors [27].
For analysis of clinical signs the men filled out internationally approved questionnaires—ADAM (Androgen Deficiency in Aging Males) [28] and AMS (Aging Male’s Symptoms) [29]. The probability (risk) for LOH is thus defined. Other reasons for hypogonadism were excluded—hyperprolactinaemia, pituitary, operative interventions and others.
The men that are to be treated with testosterone are additionally examined and contraindications for testosterone treatment are excluded. For the treatment is used either testosterone undecanoat (Nebido, Bayer-Schering) —1000 mg/4ml for intramuscular application every 12 weeks after the first interval being 6 weeks, or testosterone gel (Androgel, Laboratoires Besins International, Abbot)—50 mg in daily sachets. After starting this therapy the men are monitored for a period of three months, and blood samples are collected for further analysis.
2.3. Controls
А control group of men is formed by age-matched healthy volunteers without diabetes mellitus and/or obesity, or other known diseases that may interfere with the studied parameters. They are analyzed by the same protocol. They were told to come in the clinic in fasting state in the morning for blood drawing for laboratory analysis and routine physical examination and anthropometric measurements. After getting the results some of the men are excluded because of previously unrecognized disturbance of the carbohydrate metabolism (2), or fulfilling the criteria for MS, or low serum TT levels.
2.4. Blood Samples’ Analysis
Blood samples are taken using the forearm’s veins. Routine laboratory analyses are done in the same day. For measuring the apelin levels, after centrifugation (4000 r/min) serum samples are freezed. Further apelin levels are determined by enzyme-linked immunosorbent method (Phoenix pharmaceuticals, USA). TT-levels are measured by electrochemiluminiscent method (Elecsys 2010, Roche Diagnostics). The same method is used for measuring the levels of sex-hormone binding globulin (SHBG). Estradiol (E2) and luteinizing hormone (LH) are measured by chemiluminiscent method (ADVIA Centaur CP System—Siemens Healthcare).
2.5. Statistical Analysis
Statistical analysis of the data is done using appropriate methods for every analysis in the computer program SPSS v.17.0 (SPSS Inc., Chicago, IL, USA). Defining the type of variable distribution, we used KolmogorovSmirnoff test. Comparing the groups, we applied the Mann-Whitney test. Correlations between variables are searched using the correlation test of Pearson. Results are accepted as statistically significant if p < 0.05.
3. Results
99 men are included in the study. The patients with MS (MS-group) are 65 and the control (C) group—34. The two groups are similar by age (MS = 50 ± 10 years vs. C = 51 ± 6 years) and height (174.8 ± 6.9 cm vs. 175.5 ± 7.0 cm), but differ significantly by their weight (102.1 ± 26 kg vs. 79.1 ± 8.7 kg; p < 0.001), BMI (33.3 ± 7.7 kg/m2 vs. 25.7 ± 2.4 kg/m2; p < 0.001), waist circumference (111.7 ± 13.9 cm vs. 89.8 ± 8.2 cm; p < 0.001), hip circumference (109.8 ± 14.5 cm vs. 97.4 ± 5.5 cm; p < 0.001), waist-to-hip (W/H) ratio (1.02 ± 0.04 vs. 0.92 ± 0.06; p < 0.001) respectively. The results are presented in Table 1.
In the MS group 74% of men have previously been diagnosed with T2DM. They have mean glycated haemoglobin level—7.6% ± 1.2% and this diagnosis has been set 6.44 ± 6.52 years ago. Measuring plasma glucose during the day (at 8-, 12-, 15-, 22-h) we estimate mean daily level of blood sugar as 8.5 ± 2.7 mmol/l. In 77.5% of the men diabetic polyneuropathy has already been diagnosed. 26.5% of the diabetic men have diabetic retinopathy and in 30% it is proliferative. Nephropathy is found only in 2% of diabetics.
The patients without T2DM have immune-reactive insulin (IRI)—19.9 ± 10.5 mU/l and the calculated HOMA = 3.9 ± 1.9.
Hypertension is part of the MS in 71% of patients, and 98% of them have already been assigned to antihypertensive medication. The rest of them are prescribed to appropriate medication in the clinic. The mean level of the measured SBP is 133.4 ± 17.9 mmHg; and for DBP 82.4 ± 13.7 mmHg. 32% of the men in the control group are hypertonics.
The diagnosis dyslipidemia has already been made in 25% of patients with MS and therapy has been assigned in 81% (statin) and 19% (fibrate). After measuring lipid parameters 53% of men have low HDL, 53% have high TG, and 27.3% have both lipid abnormalities. In the control group 26% of men have low HDL, 23% have high TG and 9% have both abnormalities; only 3% of them have previously been diagnosed with dyslipidemia.
Low TT levels are found in 21 (32.3%) of men in the MS-group, and the rest 44 (67.7%) have TT-levels above the accepted cut-off level of 10.4 nmol/l. Comparing the two subgroups in the MS-group, the men with low TT tend to have higher weight, BMI, larger waist circumference and more fat mass. There is significant difference

Table 1. Characteristic of the study groups.
between the levels of LH (p < 0.05), but there isn’t such one comparing their E2. Significant difference is found comparing TT-levels of the MS-group and the controls (p < 0.001), but such is not found for E2 and LH.
No correlation is found between TT and the age of patients in the MS-group. Such is found for TT and BMI (Pearson’s = −0.349, p < 0.001), weight (Pearson’s = −0.428, p < 0.001), waist circumference (Pearson’s = −0.304, p < 0.05), fat percent (Pearson’s = −0.409, p < 0.01), HOMA index (in patients without T2DM) (Pearson’s = −0.589, p < 0.05), total cholesterol (Pearson’s = −0.289, p < 0.05), HDL-levels (Pearson’s = 0.26, p < 0.05). Data are shown in Table 2. Correlation isn’t found for the number of components of MS, duration of T2DM after diagnosis, HbA1c, LDL, and TG. The same correlations persist using the calculated levels of FT and BT, excluding that with HDL and HOMA. High correlation is found for the TT and SHBG (Pearson’s = 0.533, p < 0.001).
The questionnaire used in the study—ADAM scores the androgen deficiency in aging males. When three or more of the answers of the ten questions are positive, it is considered possible for the TT levels to be low in that patient. Our results show high correlation of the TT with the number of positive answers (Pearson’s = 0.912, p < 0.001). This correlation is preserved using FT or BT levels. The additional questions about morning erections and erection when watching erotic movie scenes, also show high correlation with the TT (Pearson’s = 0.533, p < 0.001 and Pearson’s = 0.825, p < 0.001). The other questionnaire—AMS that also refers to the symptoms of LOH, didn’t show correlation with the TT in our study, neither did its part concerning the sexual function.
Apelin levels show in our study significant difference between the MS (1.61 ± 0.53 ng/ml) and the control group (1.38 ± 0.57 ng/ml, p < 0.05), as well as between the subgroup with normal TT in the MS group (1.65 ± 0.53 ng/ml) and the controls (p < 0.05). Analyzing the subgroup with low TT in the MS, the difference with the control group is insignificant. There isn’t significant correlation with the TT or with the FT levels in the different groups.
Significant correlations with apelin levels show other laboratory parameters:
• LDL-levels in the MS-patients (Pearson’s = 0.311, p < 0.05), but not in the control group;
• HbA1c in men with T2DM (Pearson’s = 0.285, p < 0.05), but not with the duration of the disease;
• serum creatinine levels measured only in the MS patients (Pearson’s = 0.257, p < 0.001), but not with the calculated creatinine clearance.
We have looked for significant difference between apelin levels in MS-patients on metformin therapy (55%) and the rest in the MS-group, but such isn’t found.
Of the patients that are assigned to testosterone replacement therapy (TRT), only three end the monitoring period, and the rest don’t mainly due to personal (mostly financial) motives. For this reason statistical analysis is not possible. One of the three patients is treated with the intramuscular injection—Nebido, and the other two with the transdermal gel—Androgel. They all have T2DM, without changing their dietary habits and therapy during the monitoring period. We establish that TT raises to physiological levels (Figure 1); two of the men lose
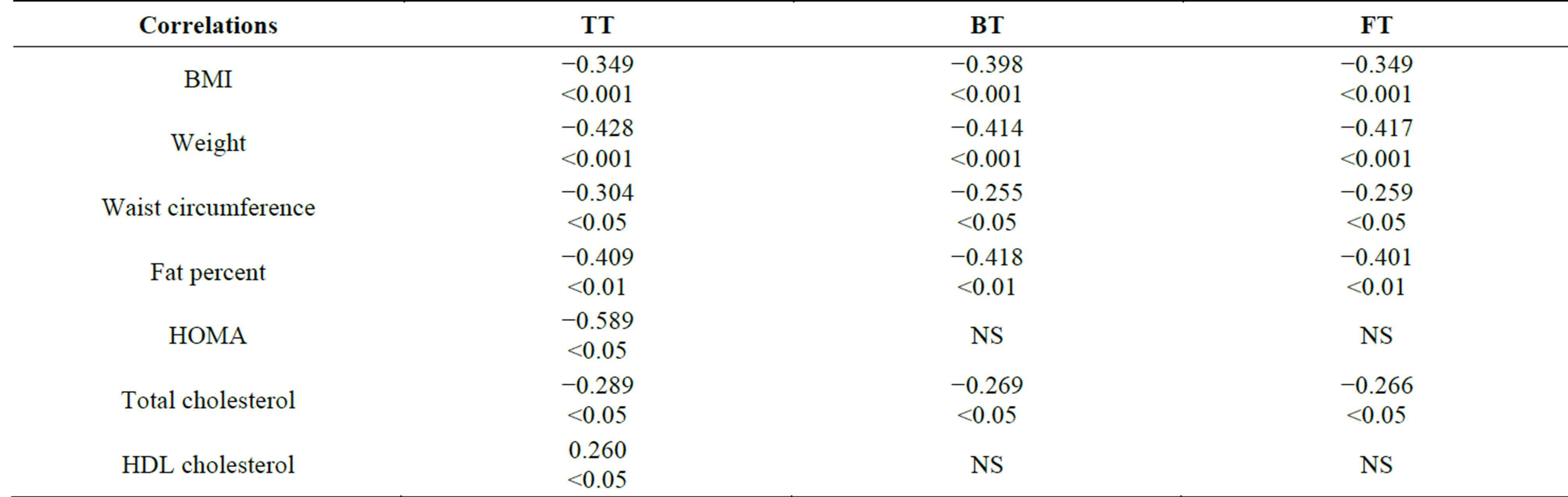
Table 2. Correlations of testosterone levels and other parameters. Data shown as Pearson’s coefficient and p value.
weight and the third doesn’t change it but does lower his fat mass, raising the lean body mass; TG after treatment are slightly lower and the total cholesterol-slightly higher; the results in the questionnaires about self-reported symptoms of androgen deficiency improve (ADAM, AMS). In all three men we establish a trend for lowering apelin levels (Figure 2).
4. Discussion
Our hypothesis in this study was that because of its tight connection with MS, LOH would influence apelin levels that are viewed to be related to the insulin resistance (IR) in the literature. Additionally we assumed that testosterone substitution would normalize apelin levels. As far as we know, this is the first study analyzing apelin, metabolic syndrome and LOH.
Our results confirm significantly higher apelin levels in men with MS compared to the controls. This is in line with the results of Yu et al. [30]. In their study, they analyze patients with T2DM and obesity and reveal significantly higher apelin levels in the patients compared to the control group. Furthermore, apelin levels are lower after treatment with insulin sensitizers (metformin and pioglitazone). Our data do not present significant correla-

Figure 2. Apelin levels during T treatment.
tion with BMI, as showed in other studies too [1,31,32]. Probably the reason for this is that fat tissue is not the only place of apelin synthesis, and other sources like the vascular endothelium could mask lesser secretion from there [33-35]. On the other hand, lower expression of apelin is demonstrated after weight loss [36].
We don’t find correlation between apelin, IR and hyperinsulinaemia, as some other studies reveal [30,31, 36-40]. This connection is ambiguous as higher apelin levels may be a result of IR, or a reason for it. It is established that hyperinsulinaemia leads to higher apelin secretion [1]. The same authors find that insulin levels are significantly lower when apelin expression is lesser in an animal model of T1DM, induced by streptozotocin. Dray et al. [35] find better glucose utilization in peripheral tissues after apelin injection that leads to decreased IR. Other authors reveal that apelin injection inhibits glucose-dependant insulin secretion in mice [41]. Additionally lowering IR is independently associated with higher apelin levels [42].
We find correlation of serum apelin levels and glycated HbA1c. Some authors propose that apelin is associated more with T2DM, than with obesity [43]. Raised apelin levels could be a compensatory mechanism to insulin resistance, which is in the basis of this condition.
Additionally there are data showing increased apelin levels after treatment with metformin and rosiglitazone and better glycemic control [44]. It is reported that apelin levels are unchanged in obese men with nonalchoholic steatosis without DM and hypertension [32]. Endogenous apelin may be insufficient or uncapable of reacting to the IR in diabetes or severe IR-state [43].
The literature analized gives contradictory data about apelin and IR. Limitation is the use of HOMA index to define insulin resistance as the golden standart is using euglycemic hyperinsulinaemic clamp technique. This could possibly compromise the results. A reason for the ambiguous data could be the different criteria and design in the studies.
Our work reveals significant correlation of apelin and LDL-C in men with MS. Tasci et al. [45] report 4-fold raise in apelin levels after 12-week programme of changing lifestyle (diet and physical activity). They examine patients without diabetes or obesity, with dislipidaemia that do not use statin treatment. The participants that achieve target level for LDL-cholesterol, have also their apelin level raised. It is established that reduction of LDL-cholesterol is an independent predictive factor for the change of apelin levels in higher physical activity [42]. The same authors report no significant difference between apelin levels in patients with DM2 and normal weight, with sedentary or active lifestyle; as the subgroups with overweight and obesity show such [42].
The etiopathogenesis of diabetic retinopathy (DRP) is not well studied. Numerous pathogenetic mechanisms are involved, one of which is the oxidative stress [46,47]. The oxidative stress plays an important role in the pathogenesis of the microvascular diabetic complications, decreasing the bioavailable nitric oxide (NO) [48,49]. Apelin lowers blood pressure through a NO-mediated mechanism [11,50]. Yonem et al. report no difference in apelin levels between patients with DRP and healthy controls [51]. Our study does not show relation of apelin and DRP.
There is a positive correlation between apelin and serum creatinine levels. This supports the established correlation with the albumin/creatinine ratio in urine sample [51]. It is reported that higher oxidative stress and lower bioavailable NO play role in the pathogenesis of diabetic nephropathy [48,49]. Zhong et al. [52] report diminished expression of apelin receptor and enhanced contractile response to angiotensin II in the renal arteries of diabetic mice. Apelin supplementation restores the normal vascular response. Possibly the correlation with serum creatinine may be explained by some compensatory and beneficial effect of apelin system on the pathogenesis of diabetic complications [51].
Studies in prepubertal children and during puberty show different correlation of apelin and obesity [33,53]. Reinehr et al. assume that a reason for this could be the sex hormones and possibly their effect on the apelin levels. The aim of our work is to look for a connection of apelin levels and sex hormones in men with MS. Our results show significant difference between the apelin levels in the group with MS and also the subgroup with normal TT levels compared to the control group. While the subgroup with low TT has apelin levels that are not significantly different from that of the controls or the subgroup with MS and normal TT level. As far as we know this is the first study concerning that interaction. Although the group with MS and the control group differ significantly by their serum apelin levels and by their TT, correlation between the last isn’t found. Anyway, the patients that are treated with testosterone, tend to have lower apelin levels after treatment. It is possibly a result of changing the body composition and weight during therapy. It is established that apelin supplement raises adrenocorticotropic hormone (ACTH) and serum cortisol and also lowers prolactine, LH and FSH up to 30 minutes after infusion in rats [13]. Our data do not show relation of apelin and LH-levels.
The part of this study focused on the effect of TRT on apelin levels may be considered only as a pilot one because of the very small number of patients. In all three of them a tendency of apelin to decline was observed. Obviously, further larger studies are needed to clarify the effect of TRT on apelin levels and the mechanisms involved in this interaction. This study has also other limitations mainly due to the number of patients.
5. Conclusion
In this study, higher apelin levels are found in the presence of MS compared to healthy men, but do not differ between men having MS with low or normal T.
Acknowledgements
This research was sponsored by Medical University— Sofia, grant № 27-D/2011 according to contract № 25-D.
Abbreviations
ACTH—adrenocorticotropic hormone
ADAM—androgen deficiency in aging males
AMS—aging male’s symptoms
APJ receptor—G protein-coupled receptor which binds apelin
BMI—body mass index
BP—blood pressure
BT—bioavailable testosterone
C—control group
DBP—diastolic blood pressure
DM—diabetes mellitus
DRP—diabetic retinopathy
E2—estradiol
FSH—follicle-stimulating hormone
FT—free testosterone
HbA1c—glycated haemoglobin
HDL— high density lipoproteins
HOMA—homeostatic index of insulin resistance
LH—luteinizing hormone
IDF—International
Diabetes Federation
IR—insulin resistance
IRI—immune-reactive insulin
LDL—low density lipoproteins
LOH—late-onset hypogonadism
MS—metabolic syndrome
NO—nitric oxide
NS—non significant
SBP—systolic blood pressure
SD—standard deviation
SHBG—sex-hormone binding globulin
T—testosterone
TG—triglycerides
TRT—testosterone replacement therapy
TT—total testosterone
T1DM—type 1 diabetes mellitus
T2DM—type 2 diabetes mellitus
NOTES