1. Introduction
With the ever increasing demand of energy and to create a sustainable world, alternative energy sources are needed. Alternative sources include solar power, biomass, wind power, harvesting energy from waves and tides and thermoelectric (TE) materials that convert heat into electricity. Thermoelectric (TE) energy conversion is a promising technology for electrical power generation by waste heat recovery. Thermoelectric materials that can convert heat energy into electrical energy directly via Seebeck effect and vice versa by Peltier effect have gained increased attention recently [1]. For a specific material, thermoelectric figure of merit (ZT) is defined as ZT =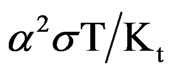 , where T is the temperature, α is the Seebeck coefficient or thermo electrical power, σ is the electrical conductivity and κt is the thermal conductivity. The compounds like Bi2Te3, PbTe and SiGe alloys are high performance thermoelectric materials with ZT > 1 [2]. These materials are not stable at higher temperatures and hence cannot be used for high temperature thermoelectric generation. For high temperature applications, oxide materials are the most suitable candidates owing to their benign nature, availability, cost effectiveness, oxidation resistance and stability [3]. Cobalt oxides are of particular interest as TE materials because of its large Seebeck coefficient and semiconducting or metallic electric conductivity [4-6]. They exhibit a strongly correlated electron system with Co ions presenting an energy level degeneracy of electronic states which are considered as the origin of the large Seebeck coefficient. Owing to their interesting electrical and magnetic properties, rareearth cobalt oxides, RCoO3 (R-rare-earth element), have been extensively studied for their thermoelectric properties [7-9]. Bismuth can be incorporated to modify the properties of alloys and metallurgic additives, thermoelectric and ferroelectric materials [10]. Atomic vibration frequencies are reduced in compounds composed of heavy elements. This will lead to lowered thermal conductivity [11]. The effective ionic radius of Bismuth is 1.03A0 which is large compared to that of Cobalt (0.545Å) and Nickel (0.69 Å). So Bismuth cobalt oxide is expected to possess low thermal conductivity, which may help to increase the thermoelectric figure of merit. The thermoelectric studies of Bismuth doped cobaltates are rarely found in literature. In the present study, the thermoelectric properties of BiCoO3 and the effect of Nickel substitution in bismuth site are reported.
, where T is the temperature, α is the Seebeck coefficient or thermo electrical power, σ is the electrical conductivity and κt is the thermal conductivity. The compounds like Bi2Te3, PbTe and SiGe alloys are high performance thermoelectric materials with ZT > 1 [2]. These materials are not stable at higher temperatures and hence cannot be used for high temperature thermoelectric generation. For high temperature applications, oxide materials are the most suitable candidates owing to their benign nature, availability, cost effectiveness, oxidation resistance and stability [3]. Cobalt oxides are of particular interest as TE materials because of its large Seebeck coefficient and semiconducting or metallic electric conductivity [4-6]. They exhibit a strongly correlated electron system with Co ions presenting an energy level degeneracy of electronic states which are considered as the origin of the large Seebeck coefficient. Owing to their interesting electrical and magnetic properties, rareearth cobalt oxides, RCoO3 (R-rare-earth element), have been extensively studied for their thermoelectric properties [7-9]. Bismuth can be incorporated to modify the properties of alloys and metallurgic additives, thermoelectric and ferroelectric materials [10]. Atomic vibration frequencies are reduced in compounds composed of heavy elements. This will lead to lowered thermal conductivity [11]. The effective ionic radius of Bismuth is 1.03A0 which is large compared to that of Cobalt (0.545Å) and Nickel (0.69 Å). So Bismuth cobalt oxide is expected to possess low thermal conductivity, which may help to increase the thermoelectric figure of merit. The thermoelectric studies of Bismuth doped cobaltates are rarely found in literature. In the present study, the thermoelectric properties of BiCoO3 and the effect of Nickel substitution in bismuth site are reported.
2. Experimental
Bi CoO3 and Ni0.5Bi0.5CoO3 samples were prepared by solid state reaction followed by normal sintering. The powders of cobalt oxide (Co3O4), nickel oxide (NiO), and bismuth oxide (Bi2O3) with purity = 99.99% were mixed according to the stoichiometric ratio and finely ground using an agate mortar and pestle to get homogeneous mixture. These well mixed powders were calcined at 700˚C for 6 hours in air and grinded again for 3 hours. The resultant powders were compacted in to disc shaped samples of diameter 10 mm and 2 mm thickness followed by sintering in air at 790˚C for 12 hours. The phase structures were investigated by X-ray diffraction (XRD) at room temperature with a Rigaku X ray powder difractometer using CuKa radiation. The micro structural features and composition were studied using a scanning electron microscope having (EDAX) capability. The Seebeck coefficient and electrical resistance data were recorded simultaneously as a function of temperature from 300˚C to 700˚C in a helium atmosphere using a Seebeck coefficient/electric resistance measuring system (ZEM-3, Ulvac-Riko, Japan). For the measurement of thermal conductivity rectangular bar shaped pellets (10 × 3 × 3 mm3) made under high pressure were used. The measurement is done using a standard thermal conductivity measurement set up employing steady state method. A temperature gradient is maintained in the sample in an inert atmosphere and the temperatures are recorded using standard thermocouples. All efforts have been made to reduce the porosity of the samples by applying sufficiently high pressure.
3. Results and Discussion
The Xray diffraction pattern of BiCoO3 and Ni0.5Bi0.5CoO3 obtained at room temperature is shown in Figure 1. Results of indexing and refinement of XRD patterns indicate the presence of a single-phase polycrystalline structure for the synthesized materials. The X-ray patterns confirm the existence of cubic structure with the reflection arising from various planes indicated in Figure 1. The samples have good crystallinity and can be indexed with ICDD (PDF-2/Release 2012 RDB 00- 049-1760). The prepared samples correspond to 197 I 23 space group. The cobalt ion is replaced by bismuth randomly at the octahedral 24 fsites. The XRD pattern also indicates that there are no obvious impurity phases in the synthesized samples. The substitution of bismuth with Nickel produces a shift of diffraction planes towards higher 2θ. This shift indicates that a lattice contraction has happened due to the substitution of bismuth with nickel. This could be attributed to the fact that the radius of nickel ion is less than Bismuth ion. The detailed structural parameters are given in Table 1.
The SEM micrographs of the samples revealed the crystallites are of micrometer dimension (Figures 2 and 3). The photograph also showed micro sized crystallites

Figure 1. Room Temperature XRD of the samples.

Table 1. Characteristics and properties of the compounds.
along with agglomerated particles. The EDAX spectrum confirmed the presence of constituent elements and weight and atomic percentage of the constituents had good agreement with the stochiometry of the prepa.
Figure 4 shows the temperature dependences of Seebeck coefficients. The sign of the Seebeck coefficient was positive in the measured temperature range, indicating that the major conduction carriers are holes. The Seebeck coefficient of BiCoO3 is high compared to Ni0.5Bi0.5CoO3. The maximum value of Seebeck coefficient shown by Ni0.5Bi0.5CoO3 was 350.5 μV/K at 760 K. Seebeck coefficient decreases rapidly with further increase in temperature. Nickel substitution in the Bismuth sites lowers the Seebeck coefficient as depicted in Figure 4. The Co3+ ions in the compound are in the low spin ground state configuration with S = 0 at room temperature, and then experience a transition to an intermediate spin state with increasing temperature. When the temperature is high enough, the Seebeck coefficient is determined by the Heikes’ equation [12]. The general expression has been given as
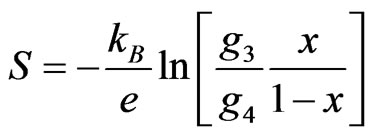 (1)
(1)
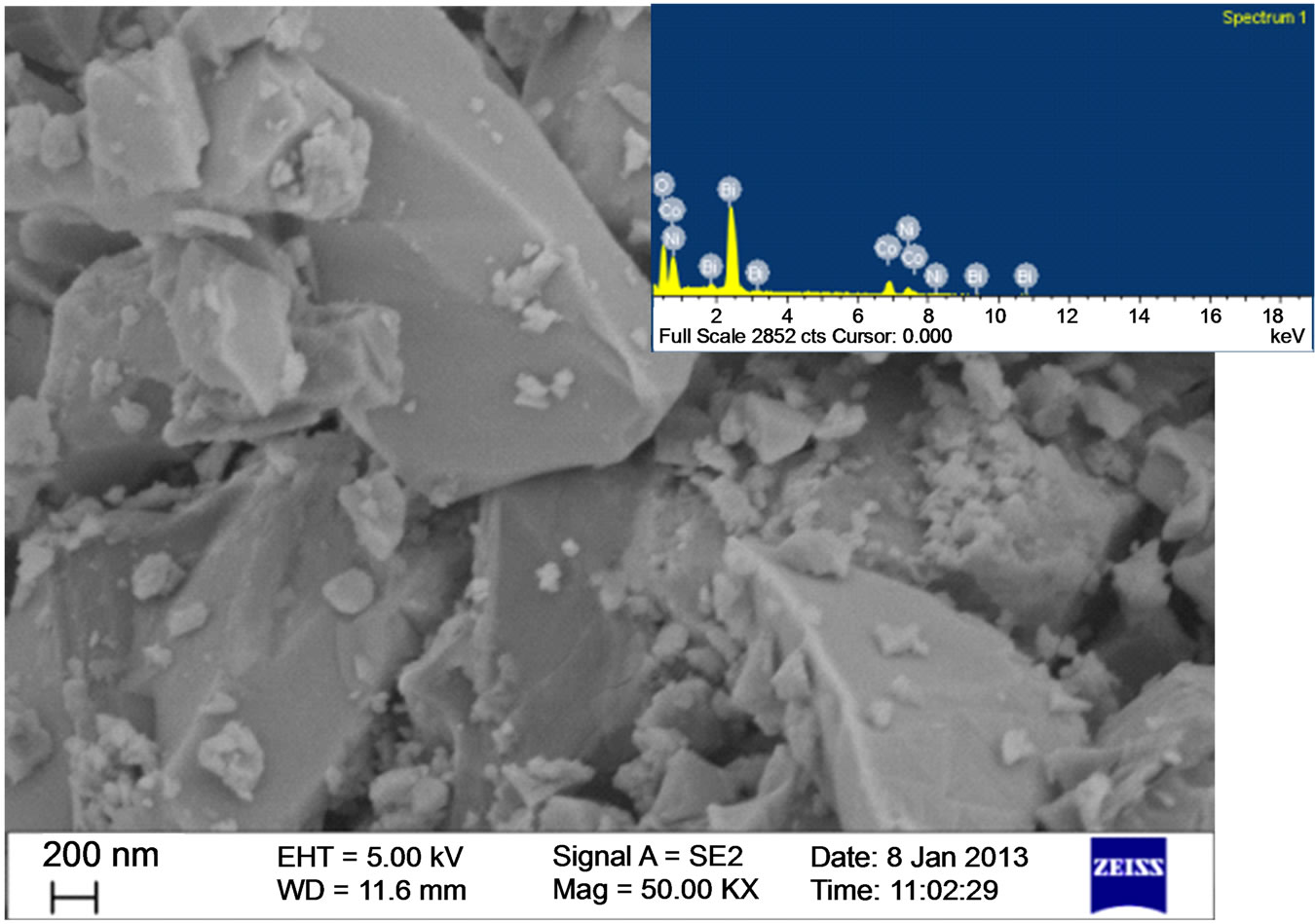
Figure 3. SEM and EDAX of Ni0.5Bi0.5CoO3.
where kB is the Boltzmann’s constant, and x is the concentration of Co4+ ions, g3 and g4 denote the degeneracy of Co3+ and Co4+, respectively, in the octahedral coordination. This indicates that the absolute Seebeck coefficient depends on the degeneration of electronic states of Co3+ and Co4+ ions as well as the ratio between them [12,13]. In BiCoO3, the substitution of Bi3+ by Ni2+ lead to an enhancement of the hole concentration and a reduction of the Seebeck coefficient. Therefore, it is inferred that Ni3+ turn to Ni2+ during sintering, since the latter is much stable at high temperatures in air [14]. The excess negative charge introduced by Ni2+ doping to BiCoO3 is compensated either by creation of holes, that is, by the oxidation of Co3+ to Co4+, or by creation of oxygen vacancies [15]. With increasing temperature, the spin state transition and the ratio of Co3+ and Co4+ would be changed, causing the reduction of the Seebeck coefficient.
Figure 5 shows the temperature dependence of electrical conductivity of the samples. The samples show semiconducting behavior in the measured temperature range as the electrical conductivity increases with increasing temperature. The substitution of Nickel with bismuth causes a noticeable increase in the electrical

Figure 4. Seebeck Coefficient of the samples as a function of temperature.
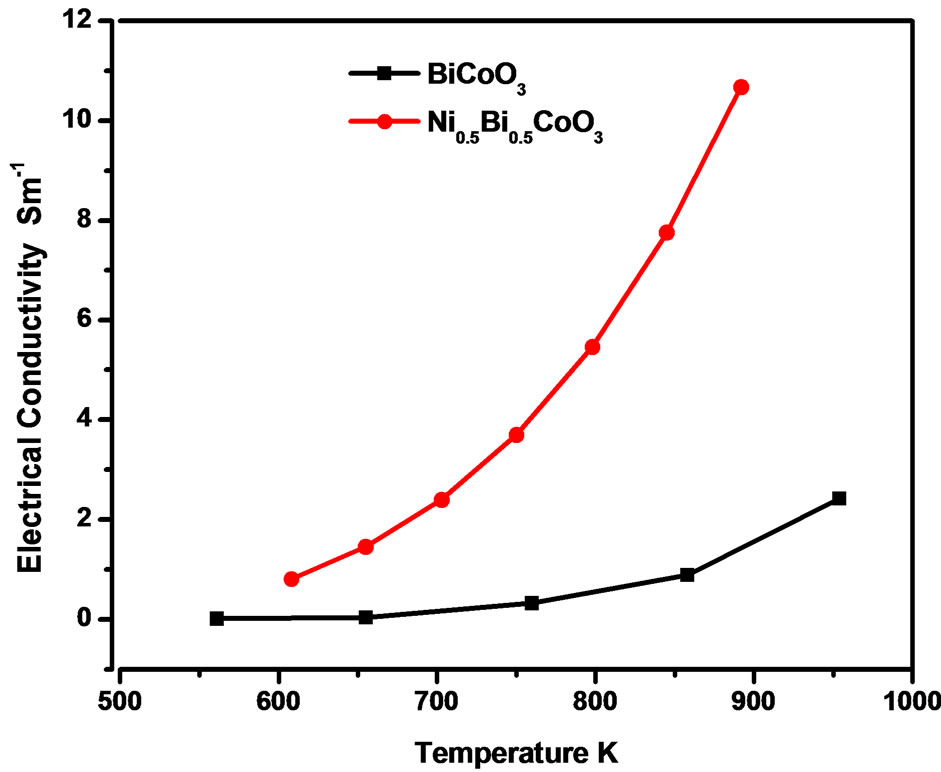
Figure 5. Electrical conductivity as a function of Temperature.
conductivity. The improved electrical conductivity is due to increased carrier concentration because of the substitution of Bi3+ by Ni2+. It is well known that the hopping conduction behavior exists in cobaltates at high temperature [16,17].
Electric conductivity
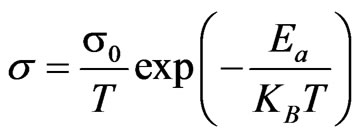 (2)
(2)
where σ0 is a constant Ea denotes the activation energy.
As shown in Figure 6, plot of LnσT versus 1/T lie on a straight line in the case of Ni0.5Bi0.5CoO3 indicating the hopping conduction mechanism. But for BiCoO3 the plot is not a perfect line indicating that the sample does not obey such transport mechanism in the measured temperature range. This nonlinear behavior of LnσT verses 1/T indicates that normal polaron hopping conduction is not shown by the material.
The temperature dependence of thermal conductivity K is shown in Figure 7. Robert et al. [18] reported an extremely low thermal conductivity reaching 0.44 Wm−1K−1 at room temperature, for cobaltates. In the present study also the samples showed extremely low thermal conductivity for the samples. The value of K shows a decrease as temperature increases and became almost steady at higher temperatures for both the samples. The total thermal conductivity can be expressed by the sum of phonon thermal conductivity Kph and carrier thermal conductivity Kel, i.e. K = Kph + Kel. The carrier thermal conductivity Kel is estimated from Wiedemann–Franz’s law 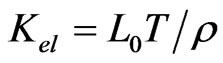 where
where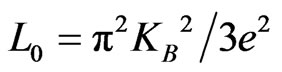 , the Lorentz constant. Kel has a very low contribution to the total thermal conductivity in the measured temperature range. Accordingly, Kph is the predominant component in K and
, the Lorentz constant. Kel has a very low contribution to the total thermal conductivity in the measured temperature range. Accordingly, Kph is the predominant component in K and

Figure 6. Plot of lnσT verses 1/T for the samples.
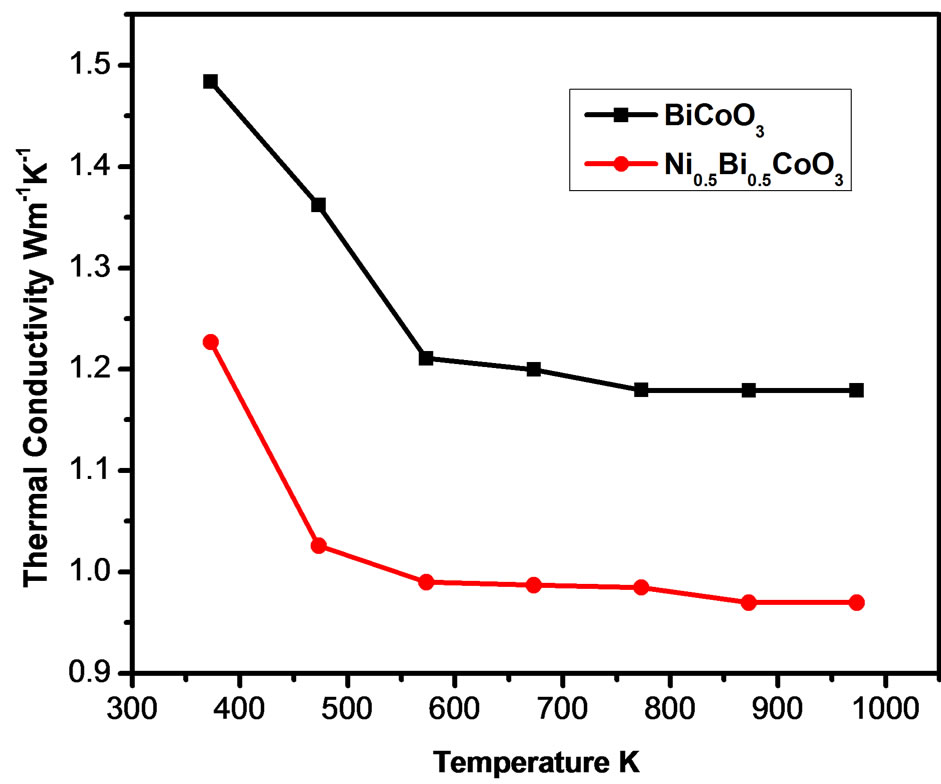
Figure 7. Thermal conductivity of the samples as a function of temperature.
the variation of K mainly arises from the alteration of Kph.. Nickel substitution brings lattice disharmony and hence strongly scatters the phonons. As a result the phonon transport is suppressed and hence K decreases due to substitution of bismuth with nickel.
The power factor (PF) is the term α2σ, and it is crucial to achieve a high PF for high performance TE generation. A high PF means that a large power output (i.e. large voltage and current) will be generated. Temperature dependences of power factor (PF) are exhibited in Figure 8. The PF of the Ni-substituted compound is larger in the measured temperature range. PF increases with increase in temperature for both the samples and the rate of increase is larger for the nickel substituted sample.
Figure 9 presents the ZT values of the samples at various temperatures. For both the samples ZT monotonously increases as temperature rises. ZT value in the nickel substituted sample was enhanced noticeably. This is attributed to the increased electrical conductivity and reduction of thermal conductivity due to the substitution of bismuth with Nickel.

Figure 8. Temperature dependence of Power factor.

Figure 9. Temperature dependence of Figure of merit.
4. Conclusion
BiCoO3 and Ni0.5Bi0.5CoO3 samples were prepared by solid state reaction and normal sintering. The samples showed crystallites of micrometer dimension. X ray diffraction studies revealed the existence of single phase with cubic structure for the samples. It is found that the substitution of Bi with Ni in BiCoO3 increases the electrical conductivity significantly, but reduces the Seebeck coefficient. For both samples, the sign of the Seebeck coefficient was positive in the measured temperature range, indicating that the major conduction carriers are holes. Ni0.5Bi0.5CoO3 showed maximum Seebeck coefficient 350.5 μV/K at 760 K. The thermal conductivity of the samples is extremely low and the nickel substitution further lowers the thermal conductivity. The figure of merit increases as temperature rises and its value in the nickel substituted sample is enhanced. Since ZT values increase with temperature, the materials can be used as high temperature thermoelectric applications.
5. Acknowledgements
The authors would like to acknowledge UGC Govt. of India, DST FIST II for the experimental and financial support. The authors are grateful to IIT Chennai for thermal conductivity measurements and Materials Engineering Department IISc. Bangalore for Seebeck coefficient measurements. T.R is thankful to UGC for award of FDP (KLCA024TF), University of Calicut and Z. G. College, Calicut for support to this work. The author PPP is thankful to DST-SERB for major project SB/EMEQ- 002/2013.