1. INTRODUCTION
Breast cancer is one of the worldwide deathly cancer type after lung, liver and colon cancer. It is the main enemy of women and currently in the first rank of danger. Identification of breast cancer cases worldwide has increased from about 640.000 in 1980 to 1.6 million by 2010 in developing country [1]. Patients with breast cancer are mainly caused by the diet, such as the selection of foods and drinks contain preservatives, coloring agents and beverages that contain alcohols [2].
Cause of cancer usually cannot be known with certainty because it is a combination of a set of factors including genetics, environment, foods containing chemicals, viruses, for example Papilloma Virus, which caused genital warts/genital, cytomegalo virus that caused Kaposi sarcoma/cancer of vascular system marked by blood red skin lesions, Hepatitis B virus, hormonal imbalance, free radicals, psychological and emotional factors [3].
The efforts for developing anticancer drugs had been continued intensively, both derived from chemicals and natural sources. Some research showed that many of the plants contain a variety of chemical compounds as potential anticancer. One of those things that has became scientists’ observation is traditional medicine. This research is done because the potential of traditional medicine has long been believed by the people to cure the disease [4].
One of the plants that can be used for the cancer treatment is ekor naga’s leaves (Rhaphidophora pinnata (Lf) Schott), from Araceae family. Some people have used the boiling water for the treatment of cancer. Polyphenolic compounds (flavonoids, tannins and saponins) are generally effective as antioxidant and could kill the growth of abnormal cells (tissue) and uncontrolled cell, which is one of the causes of cancer [5].
Pre-eliminary screening test for active compounds contained in the extract was the toxicity test on the shrimp larvae Artemia salina L. using the brine shrimp test method with LC50 values of crude ethanol extract 19.686 mcg/ml, n-hexane fraction 505.82 mcg/ml, ethyl acetate fraction 28.84 mcg/ml and ethanol fraction 128.82 mcg/ml [6-9]. Acute toxicity test with oral administration in female mice up to doses of 5000 mg/kg BW did not reveal any mortality up to 7 days and no physiological changes in the body of mice. Therefore, the LD50 value of the ethanol extract of ekor naga’s leaves cannot be calculated and it was declared the value LD50 was greater than 5000 mg/kg/BW in female mice.
One model of breast cancer cells used MCF-7 cells that had been patented by the institute of Michigan Cancer Foundation-7 (MCF-7). MCF-7 cells are cell type isolated in 1970 from a woman’s breast tissue of Caucasian race. MCF-7 cells have been used for a variety of in vitro research on breast cancer because they have some of the same characteristics associated with breast epithetlial estrogen in the form of the ability to process estradiol via estrogen receptors in the cytoplasm. It makes the MCF-7 cells give positive result to estrogen (ER) recipeent in the control cells [10].
In order to find new drugs for the treatment of breast cancer, the in vitro cytotoxicity test of the ethanol extract (crude) and fractions (n-hexane, chloroform, ethyl acetate and water) of ekor naga leaves against MCF-7 cells had been done.
2. MATERIALS AND METHODS
2.1. Materials and Reagents
The material used in this study was ekor naga’s leaves. The chemicals used were, n-hexane (Merck), 96% ethanol (Merck), chloroform (Merck), ethyl acetate (Merck), a solution of dimethylsulfoxide (DMSO) pro 99.5% GC (Sigma), Roswell Park Memorial Institute Medium (RPMI 1640), hepes (Sigma), 3-(4,5-dimetiltiazol-2-il)-2, 5-diphenyl tetrazolium bromide (MTT), Fetal Bovine Serume (FBS), Penicillin-Streptomycin, silica gel GF254, H2SO4p (Merck), methanol (Merck), acetone (Merck), Amphoterisin B, Sodium dodecyl sulphate in 0.01N HCl, Doxorubicin, MCF-7 cell line.
2.2. Phytochemical Screening
Phytochemical screening of ekor naga leaves powder, ethanol extract and fractions of class compounds included examining alkaloids, glycosides, anthraquinone glycosides, saponins, flavonoids, tannins, and triterpenoida/steroid.
2.2.1. Examination of Alkaloids
Crude leaves powder weighed 0.5 g and then diluted with 2 N hydrochloric acid and distilled water, heated over a water bath for 2 minutes, cooled and filtered. Filtrate was tested with the addition of reagent of alkaloid such as Mayer, Bauchardat, and Dragendorff.
2.2.2. Examination of Glycosides
Crude leaves powder was refluxed for 2 hours with a mixture of ethanol-water (7:3) and 2 N hydrochloric acid, then filtered. Filrate was added with a solution of lead (II) acetate 0.4 M, then shaken, and filtered. Filtrate was extracted with isopropanol-chloroform mixture (2:3). Water extract was collected and evaporated at a temperature of not more than 50˚C. The remaining part was dissolved in methanol and added with 2 ml of water and reagent Molish. Then slowly 2 ml of concentrated sulfuric acid was added through the tube wall. The formation of a purple ring was formed.
Examination of anthraquinone glycosides Crude leaves powder was heated with 5 ml of 2 N sulfuric acid, then cooled it room temperature, the mixture then was added with 10 ml of benzene, shaken and allowed to stand. Benzene layer was separated and shaken with 2 ml of 2 N NaOH, allowed stand. Water layer is red and colorless benzene layer showed the presence of anthraquinone.
2.2.3. Examination of Saponin
Crude leaves powder was inserted into a test tube, then hot water was added. The mixture was cooled then shaken vigorously for 10 seconds. If the 1 - 10 cm tall foam was formed and stable for not less than 10 minutes and did not disappear with the addition of 1 drop of 2 N hydrochloric acid, then it was positive for saponin.
2.2.4. Examination of Flavonoide
Crude leaves powder was shaken with hot water for 5 min and filtered under hot conditions, the filtrate was added magnesium powder, and concentrated with hydrochloric acid and amyl alcohol, then shaken. It showed flavonoid with positive if a red or yellow or orange colour in the lining of amyl alcohol was formed [11].
2.2.5. Examination of Tannin
Crude leaves powder was boiled in water for 3 minutes, cooled and filtered. The filtrate was added with 1 - 2 drops of 1% iron (III) chloride. If there is blue-black or blackish green indicates the presence of tannins [11].
2.2.6. Examination of Steroid/Triterpenoida
Crude leaves powder was macerated with 20 ml n-hexane for 2 hours, then filtered. Filtrate should was evaporated in the evaporating dish, then added with a few drops of Liebermann-Burchard reagent. Onset of blue or blue-green color showed the presence of steroid, while the color is red, pink or purple that indicated triterpenoid [12].
3. PLANT MATERIAL AND EXTRACTION OF EKOR NAGA’S LEAVES
Extracts were performed using 96% ethanol by percolation method [13].
Dried ekor naga’s leaves were weighed approxinately 900 g and soaked with n-hexane for 24 hours to eliminate the fat content. The residue was filtered and aerated until the n-hexane solvent evaporated, then soaked in ethanol for 3 hours for percolation the mass was moved into the percolator while carefully pressed and slowly poured the solvent until the liquid began to drip and the sample was still embed in the solvent. After that the percolator was closed and left for 24 hours. Then the tap was opened and the liquid was allowed to drip at rate of 1 ml per minute. Solvent was added continuously until there was a layer of liquid on top of sample. Percolation was continued until the last out of liquid evaporated leaving no residue. Resulted liquid was evaporated using rotary evaporator with temperature ±40˚C, and then freeze dried until highly viscous extract was obtained as much as 138 g. Proceed the fractionation by liquid-liquid extraction.
3.1. Liquid-Liquid Extraction of Ethanol Extract
Fractionation was performed using solvents with different polarity from non-polar to polar such as: n-hexane, chloroform, ethyl acetate, and water.
A total of 100 g of ethanol extract was dissolved in ethanol and then added with distilled water, the mixture was put in a separating funnel and added with 200 ml of n-hexane, shaken, allowed to stand until there were 2 separated layers. n-hexane layer (upper layer) was taken with decantation methode. Fractionation was done if the n-hexane layer was colorless (clear) and did not give color with Liebermann-Burchard reagent. The fractionation was continued with addition of 200 ml of chloroform in the shaker (separating funnel). The sample was shaker and then allowed to stand until there were 2 separated layers. The chloroform layer was taken (bottom layer). Fractionation was done until the chloroform was layer colorless gave negative result with Dragendorff reagent, then add 200 ml of ethyl acetate in water layer, shaken, and then allowed to stand until there are 2 separate layers. Fractionation is done until the color of the layer and the ethyl acetate is clear then fraction of water is taken, all the fractions obtained were evaporated by vacuum rotary evaporator. Each of the obtained fractions was assay for cytotoxic activity.
3.2. In Vitro Assay for Cytotoxic Activity
Five milligrams of ethanol extract and the fractions (nhexane, chloroform, ethyl acetate and water) were dissolved with the aid of 100 µL of DMSO, then diluted with cultur media in order to get a series of concentration (500, 250, 125, 62.50, 31.25 mcg/ml). The culture media was used as negative control and doxorubicin was used as positive control with series concentration of (200, 100, 50, 25 mcg/ml). Each concentration was made replication for 3 times.
3.3. Cytotoxic Assay with MTT Method
MCF-7 cells were growth in 96 wells microplate in order to obtain the density of 1x 104 cells/wells and incubated for 24 hours to get good growth. After the medium was replaced with a new one it was added with 100 µL of test solution in a variety of concentrations and incubated again for 24 hours. At the end of incubation, the media was be removed and washed with PBS solution, and then each of the wells was added with 10 µL MTT (3-(4.5-dimetiltiazol-2-il)-2.5-diphenyl tetrazolium bromide) 0.5% in PBS. Incubation was continued for 4 hours at 37˚C until formazan was formed. The living cells were converted MTT into dark-blue formazan. MTT reaction was stopped by the stopper reagent (100 µL of Sodium dodecyl sulfate), and then incubated overnight at room temperature. The absorbance was read by ELISA reader at a wavelength of 595 nm. The absorbance results were converted into percentage of life reads. MTT method was performed according by Mossman [14] with modification of stopper reagent.
3.4. Data Analysis
Data obtained in the form of absorbance values of each which converted into percentage of living cell and calculated with the following formula:

Then IC50 concentrations calculated were by probit analysis using SPSS 17, in order to obtain linearity between log concentration and percentage of live cells. IC50 is the concentration that causes cell death for 50% of the population.
4. RESULTS AND DISCUSSION
Extraction of plant material was done to get secondary metabolites from plant material which is used as medicine. Percolation extraction had been done by ethanol with obtained 117.4 g from 900 g of dried “ekor naga” leaves.
4.1. Phytochemical Screening
The identification of leaves powder and ethanol extract of ekor naga’s leaves chemical compounds were done with some color reagents to obtain the class of secondary metabolites information. The results could be seen in Table 1.
Flavonoids as antioxidants can prevent and cope with of cancer. Mechanism of action: flavonoids in cancer treatment can inhibit the cancer cells growth through the inhibition of cell cycle mechanism, induce apoptosis, inhibition of angiogenesis, antiproliferative or a combination of these mechanisms [15].
4.2. Liquid-Liquid Extraction
Solvent extraction with n-hexane, chloroform and ethyl acetate obtained 3.681 g of n-hexane fraction, 1.680 g of chloroform fraction, 0.572 g of ethyl acetate fraction, and 24.809 g of water fraction. The screening results of n-hexane fraction with Lieberman-Burchard (LB) reagent gave a blue purple color, which was likely to contain triterpenoid/steroid compound. The chloroform fraction gave pink color wich may contained triterpenoid. Triterpenoid is an antioxidant as a catcher of free radicals that can kill brain cells and also could revitalize blood vessels
Ethyl acetate fraction with FeCl3 reagent gave a positive result, which was likely to contain polyphenolic compounds that can inhibit the growth of cancer cells through the inhibition of cancer cell growth, induce apoptosis, inhibition of angiogenesis, antiproliferative or a combination of these mechanisms [15].
4.3. Assay Anticancer
The results of the extract and some fractions treatment on MCF-7 cells can be seen in Figure 1. The morphology of MCF-7 cell due to the treatment of n-hexane and water fractions did not provide significant changes to the untreated cells (control cells). While the ethanol extract (crude), chloroform and ethyl acetate fractions with high concentration, i.e. 500 mcg/ml could be seen dead cells which was round (Figure 1).
Cytotoxicity assay were performed to confirm the cy-
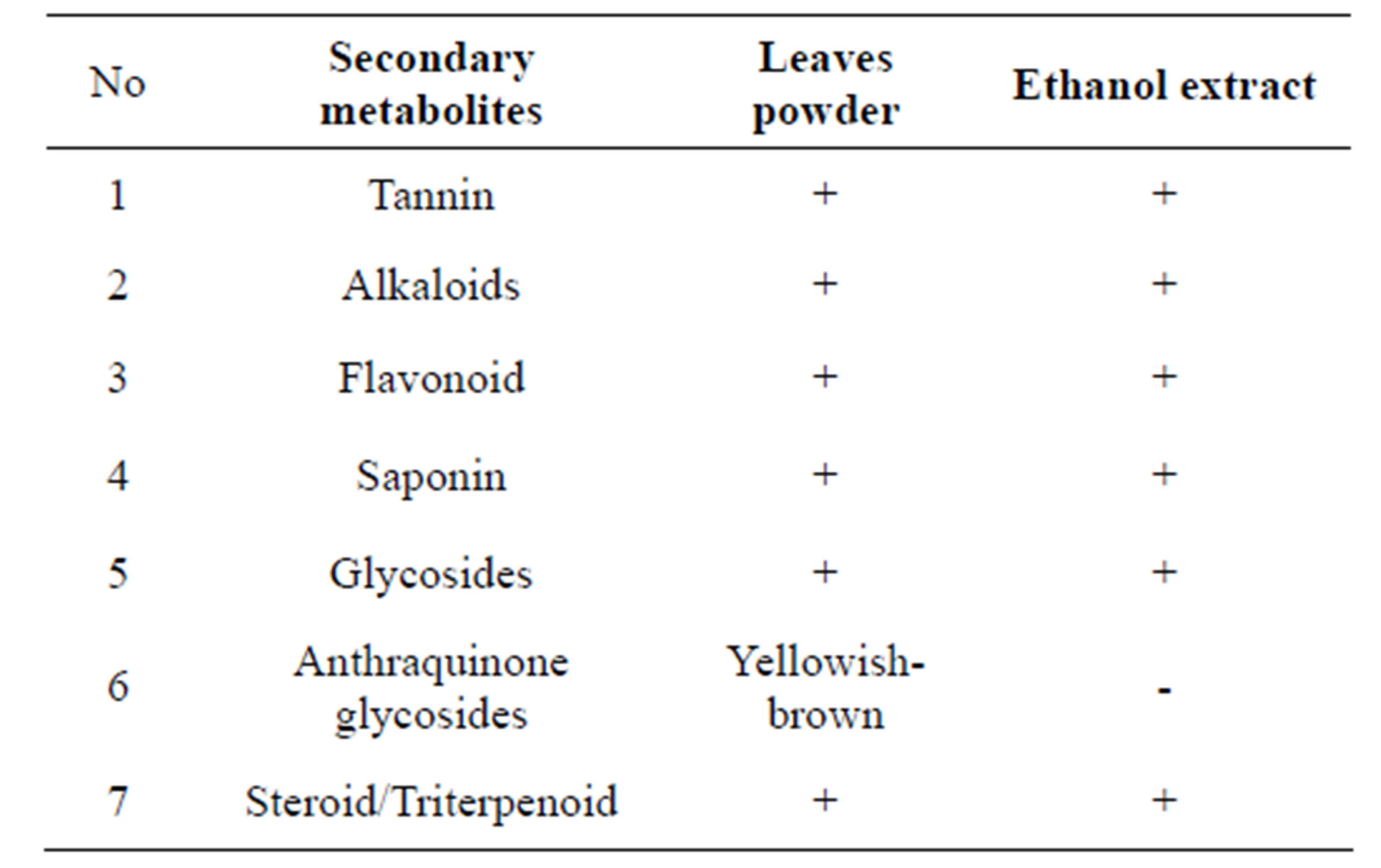
Table 1. Phytochemical screening simplex and ethanol extract.
totoxic ability of the sample solution against MCF-7 cells with a quantitative colorimetric method based on the measurement of the color intensity that occurred as a result of metabolism of a substrate by living cells into colored product. This study used yellow MTT {(3-(4,5-dimetaltiazol- 2-il)-2,5-diphenyl tetrazolium bromide} to form a dark blue formazan which insoluble in water and attached to the cells. The ability of the succinate dehydrogenase enzyme to reductase tetrazolium system were included in the mitochondria of living cells [14,16]. The method was fast, sensitive, accurate and could be used to test large samples which automatically using a spectrophotometer [16], that also proved more reliable than cell counting using a hemocytometer [17].
The results of t-test analysis of the sample treatment with several concentrations, positive control, control cells, and media control can be seen in Figure 2.
From Figure 2, it was known that the ethanol extract (concentration of 31.25 - 250 mcg/ml). The chloroform fraction (concentration of 31.25 - 500 mcg/ml), ethyl acetate fraction (concentration of 31.25 - 500 mcg/ml), n-hexane fraction (concentration of 500 mcg/ml) and water fraction (concentration of 62.5 - 500 mcg/ml), showed a significant difference (p < 0.05) to the positive control, media control and control cells.
Cytotoxic test gives an overview of the potential test-

Figure 1. The effect of ethanol extract (A), hexane fraction (B), chloroform fraction (C), ethyl acetate fraction (D), and water fraction (E) of ekor naga’s leaves on MCF-7 cells after incubation for 24 hours. The observation of cell morphology at 24 hours was performed using an inverted microscope with 400× magnification. Living cells were indicated by black arrows, whereas dead cells were indicated by red arrows.
ing material in inhibiting cell growth. The parameters used are the 50% of inhibition concentration (IC50). The smaller value of IC50 the more potential in inhibiting cell growth, as Figure 3 shows chloroform fraction IC50 was smaller than ethanol extract and ethyl acetate fraction.
Doxorubicin (Adriamycin) is used as a positive comparator and a chemotherapeutic agent against MCF-7 cells because it is currently one of anti-tumor chemotherapy drug that had been using successfully and extensively, although the side effects will increase with the increase the dose. Based on the results of the research, it was known that it has cardiotoxic effects associated with cumulative dose [18,19]. In addition beside it is toxic against normal tissues, it was also known as the cause of tumor cell that resistance to drugs, such as resistance of MCF-7 cells [20].
IC50 analysis results indicate that a parameter for cytotoxic potential of a test substance using probit analysis SPSS 17, was calculated from the relation between different concentrate with the percentage of live cells resulted ethanol extract IC50 112. 240 mcg/ml, chloroform fraction IC50 59. 082 mcg/ml, ethyl acetate fraction IC50 812. 663 mcg/ml, while the fraction of n-hexane and

Figure 2. Effects of samples treatment to of the MTT absorbance with different concentrations. Description: 1. Extract concentration 500 mcg/ml; 2. 250 mcg/ml; 3. 125 mcg/ml; 4. 62.5 mcg/ml; 5. 31.25 mcg/ml; 6. positit control; 7. media controls; 8. control cells.

Figure 3. IC50 results with some samples Description: IC50 of n-hexane and water fractions is not detected.
water didn’t inhibit the growth of MCF-7 cells. Doxorubicin has IC50 of 1.989 mcg/ml, when compared with doxorubicin, the samples still had lower IC50 concentration sample, which can be used to combine the sample fraction that had smaller IC50 with doxorubicin which can reduce the effect of cardiotoxic and resistancy of MCF-7 cells.
5. CONCLUSION
The IC50 values of ethanol extract obtained by cytotoxic assay results was 112.240 mcg/ml, chloroform fraction was 59.082 mcg/ml, and ethyl acetate fraction was 812.663 mcg/ml, while the fraction of n-hexane and water did not inhibit the growth of MCF-7 cells.