Protein Globule Formation in the Liver Graft during Cold Preservation for Liver Transplantation: Its Clinical and Pathological Significance ()
1. Introduction
Liver graft Ischemia-Reperfusion Injury (IRI) occurs during the cold preservation and reperfusion of the liver graft prior to transplantation into the recipient [1-4]. This IRI includes lesions such as fatty degeneration, which may affect the recovery of graft function. As such, it is necessary to evaluate the condition of the liver graft before transplantation. Previous research by our group has shown that eosinophilic bodies emerged in liver lobes during liver transplantation [5]. These eosiniphilic bodies (or “protein globules”) had not been reported previously in the literature. To further investigate these protein globules, light microscopy and transmission electron microscopy were used to observe IRI and protein globules formed in the liver graft. Periodic Acid-Schiff (PAS) stain; immunohistochemical staining of IgG, IgM, IgA, C3d, C4d, Fib, C1q, and CD61 were used to determine the composition of the protein globules.
2. Material and Methods
2.1. Specimen Collection
A prospectively maintained clinical transplant database that includes recipient and donor demographics, clinical details, and recipient outcomes was used for this study. All of the 95 transplantations performed at Dongfang Hospital between January 1, 2007, and June 30, 2010 were reviewed and included in this research. Of the 34 transplant recipients, 29 were male and 5 female, aged from 21 to 76 years (median age 47 years). The liver recipients’ pathological diagnoses included 26 cases of hepatocellular carcinoma, 4 cases of cirrhosis with hepatitis B, 1 cases of severe hepatitis B, 1 case Wilson’s disease, 1 case of primary biliary cirrhosis, 1 case of autoimmune hepatitis. All the 34 donors were males, aging from 25 to 30 years (median age 27 years), were dead with the cause of brain trauma. All the recipients’ and corresponding donors’ blood types were matched.
There were 68 liver specimens collected from 34 liver grafts, and these were divided into two groups as experimental groups. The A group included 34 specimens harvested from 34 liver grafts when graft trimming. The B group included 34 specimens harvested from the same 34 liver grafts during liver transplantation surgery but before abdominal closure. 34 liver harvested from the recipients, 30 liver tissue with chronic hepatitis B infection, 17 surgical liver specimens with no liver lesion, 13 autopsy liver specimens with no liver lesion, and 10 liver puncture biopsies about 10 to 20 days after liver transplantation had no cold preservation injury were used in this study as control. Informed consent was obtained from each recipient and donor or their guardians. This research was approved by the ethics committee of Dongfang Hospital.
All the liver grafts were prepared by the rapid multiple donor organ procurement surgical technique, as described elsewhere [6]. Briefly, 3000 ml HC-A(hypertonic citrate purine solution) was used to flush the liver via the aorta, 1500 ml via portal vein and then 2000 ml histidinetryptophan-ketoglutarate (HTK) solution [7]. The liver grafts were stored at 0˚C to 4˚C following perfusion with 1000 ml University of Wisconsin solution [7] via the portal vein. All the perfusion pressure was 1.2 meters height. The liver graft warm ischemia time (graft ischemic time in vivo in the donor) was 5 ± 1.2 min, and the cold ischemia time was 9 ± 2.5 h. The time of liver transplantation operation was 8.2 ± 1.25 h. Methylprednisolone, FK506, and/or cyclosporine was used as anti-rejection therapy after operation [8].
2.2. Reagents
The rabbit anti-human antibodies to IgG, IgM, IgA, Fib, C3d and C1q were purchased from DAKO (Carpinteria, CA, USA). The rabbit anti-human antibody C4d was purchased from Biomedica (Austria), and the rabbit CD61, HBsAg and HBcAg were purchased from Zhongshan Golden Bridge Company (China). The EliVision TM plus broadspectrum kit (PV-9000) used for immunohistochemical staining was purchased from Maixin Bio company (China), and Trypsin 250 was purchased from Sigma (USA).
2.3. Specimen Processing and Staining
All liver tissue specimens of A and B group split into two halves,one half were fixed with 40 g/L neutral formalin, embedded in paraffin, cut serially to 3 µm,stained with hematoxylin and eosin (H&E), periodic acid-Schiff stain (PAS), Masson’s trichrome, and immunohistochemically stained to detect the expression of IgG, IgM, IgA, C3d, C4d, fibrinogen (Fib), C1q, CD61, HBsAg and HBcAg. Another half at the same time were fixed with 3% glutaraldehyde, osmium tetroxide, embedded in E-618 resin. Ultrathin sections were stained with uranyl acetate and lead citrate and examined under transmission electron microscopy.
Immunohistochemical staining was performed as previously described [9], using tissue sections embedded in paraffin that were then dewaxed. Antigen retrieval except HBsAg and HBcAg was performed by pressure cooking the treated tissue sections in citrate buffer (0.01 mol/L; pH 6.0), followed by treatment with 0.l% trypsin (IgM, IgA, Fib for 16 min; IgG for 11 min, C3d, C4d, C1q for 7 min; CD61 was not treated with trypsin), and then soaked in 3% H2O2 for 10 min. The first antibody was then added to the treated tissue sections and incubated at 37˚C for 2 h, followed by incubation of the tissue sections in a polymer enhancer at 37˚C for 30 min. Horseradish peroxidase-conjugated anti-C4d antibody for 30 m at 37˚C, AEC chromogen. The sections were washed with phosphate buffered saline (PBS; 0.01 M, pH 7.2) throughout the above steps. We used 10% non-immune rabbit serum or PBS as negative controls, hepatitis B tissue as HBsAg and HBcAg positive controls, lupus nephritis kidney tissue as IgG, IgM, IgA, C3d, C4d and C1q positive controls, and arterial thrombus tissue as the CD61 positive control.
2.4. Lesions Observation Criteria
The severity of IRI was assessed according to hepatocyte necrosis around the central vein of the hepatic lobule in zone 3 but not in the region under the liver capsule [10, 11] as follows: no necrosis was 0 points, 1 layer of liver cell necrosis around the central vein was 1 point, 2 layers of liver cell necrosis was 2 points, and ≥3 layers of liver cell necrosis was 3 points [11]. Immunohistochemical staining was semiquantitative, and assessed the area occupied by protein globules within the hepatic lobule: no protein globules were 0 points, <25% area occupied by protein globules for 1, 26% to 50% for 2, >51% for 3.
2.5. Image Analysis Measurements of Protein Globules
In the A group, the IgG stained images were analyzed with the Motic Med 6.0 image analysis system. Five fields were randomly selected from each section at 400 magnification. The diameter of every protein globule was measured.
2.6. Monitoring Graft Function
In order to eliminate the effect of recipient pre-transplant liver condition and post-transplant rejection, 26 hepatocellular carcinoma cases with no liver failure were selected to monitor the graft function after transplantation (1 to 5 days) by assessing the serum alanine aminotransferase (ALT), aspartate aminotransferase (AST) and total bilirubin (TBIL) levels.
2.7. Statistical Methods
Non-parametric variables were expressed as median and range, and were compared using the rank sum test. Count variables were compared using the Mann-Whitney test or Kruskal-Wallis H test. Differences were judged to be statistically significant when P < 0.05. Data were analyzed using SPSS version 16.0 software (SPSS 16.0, Inc., Chicago, IL).
3. Results
3.1. Lesions under the Light Microscope
All the 34 liver grafts included in this study, 20 liver specimens had a normal appearance, 6 had mild chronic hepatitis B, 8 had mild fat degeneration.
All the 34 liver graft hepatocytes in A group were mildly swelling, and hepatocytes around the central vein of hepatic lobule zone 3 displayed mild coagulative necrosis. Varying amounts of protein globules were scattered in the lobule. They were stained uniformly red or purple with H&E (Figure 1), PAS (Figure 2) or Masson’s trichrome (Figure 3), and appeared mostly clear
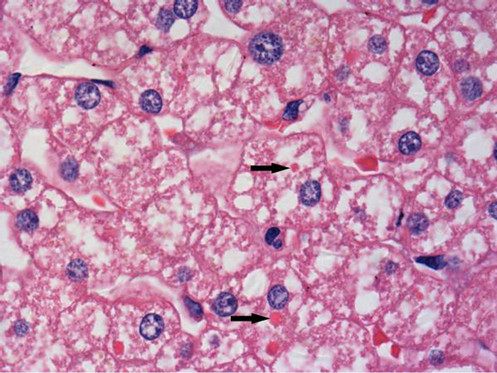
Figure 1. Protein globules (arrows) in liver graft were stained uniformly red with Hematoxylin and Eosin, more deeply stained than hepatocyte cytoplasm, but less deeply stained than RBC. Therefore these were not hepatocyte cytoplasm or fragment of RBC (×1000 magnification).
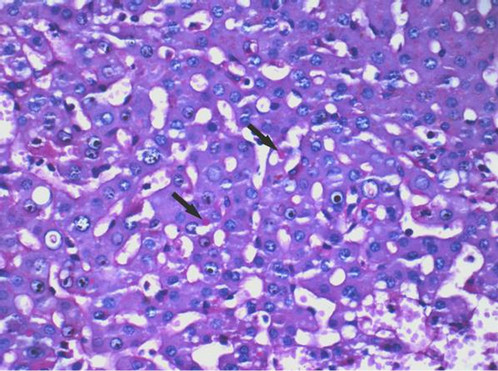
Figure 2. Protein globules (arrows) in hepatocyte cytoplasm were stained uniformly red with periodic acid-Schiff stain (PAS). Therefore these were not fragment of RBC, otherwise they should not be stained with PAS. These globules contain glycogen or polysaccharide that can be stained with PAS (×400 magnification).
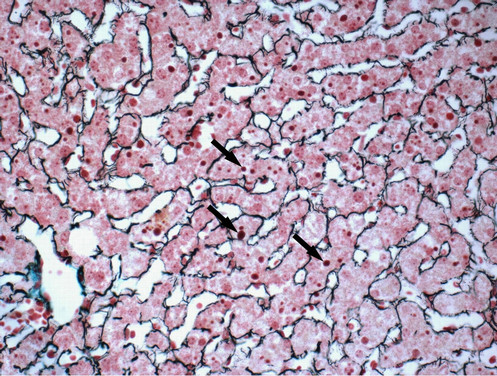
Figure 3. Protein globules (arrows) in hepatocyte cytoplasm were stained uniformly purple with Masson’s trichrome. The fibrous scaffold was stained to more clearly observe the protein globules (×400 magnification).
under Masson’s trichrome staining. The protein globules were round or oval with sharp edges, between 1.59 and 9.41 µm in diameter, and scattered in the liver sinusoids, the space of Disse or in the hepatocyte cytoplasm. The number of protein globules decreased from Zone 3 to Zone 1 in the lobule.
Hepatocyte necrosis, apoptosis and eosinophilic degeneration were all present in Zone 3. Focal bruising or bleeding with leaking of a few neutrophils and mononuclear cells, hepatic sinusoidal endothelial cell swelling, and necrosis were also observed in Group B specimens. Data presented in Table 1 shows a higher IRI average score in Group B specimens compared to Group A specimens (Table 1, P < 0.01).
3.2. Lesions in Liver Graft Specimens Observed Using the Transmission Electron Microscope
Specimens of Group A had only mild swelling of mito-
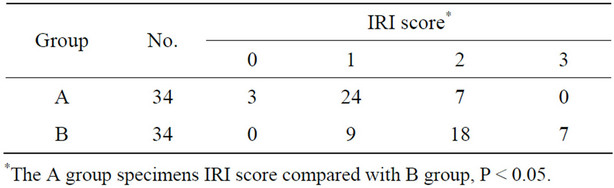
Table 1. Ischemia-reperfusion injury (IRI) of the liver graft in the A and B groups.
chondria and distribution of dense glycogen was uniform. There were also no membrane-wrapped ball bodies (Figure 4) with sharp edges in the cytoplasm of liver cells, and composition of granular material was uniform when observed with medium electron density.
In contrast to results observed in Group A, mitochondria of specimens in Group B exhibited more swelling, and became rounded or spherical in shape or ruptured in some cases; the presence of mitochondrial vacuoles also increased in Groups B compared to mitochondria observed in specimens in Group A. The number of glycolgen granules decreased resulting in a more sparse distribution in specimens in Groups B compared to those in Group A. The cellular matrix had a lower electron-density and vacuolation increased in Group B specimens compared to those of Group A. The microvillus on the micro-duct surface was swelled and the number of microvillus reduced. The nuclear membranes were disrupted, and nuclear heterochromatin reorganized into blocks and underwent shrinkage. Chromatin condensed into a half-moon or cap shape and attached to the nuclear membrane, apoptotic bodies formed, and endothelial cells contracted while their nucleus became round.
3.3. Immunohistochemical Staining Results
Immunohistochemical staining was used to identify protein globules in specimens from liver grafts. Protein globules in Groups A and B specimens had various scores of IgG (Figure 5), IgM, IgA, Fib and C3d, as assessed by immunohistochemical staining, while C4d, C1q and CD61 were negative. However, there was no significant difference in IgG, IgM, IgA and C3d scores between Groups A and B (Table 2, P > 0.05). IgG had the highest score of all the parameters assessed using immunohistochemical staining, so IgG score can present the number of protein globules. All the specimens in Group B were divided into two groups according to their IgG score less than 2 or greater than or equal to 2. There was also no significant difference in the IRI score between the IgG < 2 group and IgG ≥ 2 group (Table 3, P > 0.05). All the grafts were divided into two groups according to the time of cold ischemia: <9 h and ≥9 h. There was no correlation between the presence of this globules and the length of cold ischemia (Table 4, P > 0.05).

Figure 4. Electronic micrograph of the protein globule (arrow) in hepatocyte cytoplasm with medium electron density (×6.3 K magnification).

Figure 5. Liver specimens harvested before abdominal closure. Protein globules (arrows) in hepatocyte cytoplasm stained with IgG. Liver sinusoids were also stained for IgG. EliVision, IgG (×400 magnification).
3.4. Protein Globules and Liver Graft Function in 5 Days Post-Operation
In Group A, 26 cases which had hepatocellular carcinoma (HCC) with no liver failure were selected and then divided into two groups according to the IgG score: Group L < 2 and Group H ≥ 2. Liver function was assessed by using AST, AST and TBIL. There was no significant difference in ALT, AST and TBIL between L and H group (Figure 6, P > 0.05).
3.5. The Result of the Control Group
All the 34 recipients’ liver, 30 livers with chronic hepatitis B, 30 livers with no liver lesion did not find protein globules that found in liver graft.
4. Discussion
4.1. The Mechanism of Globulin Droplet Formation
As reported in the literature, there are two forms of liver
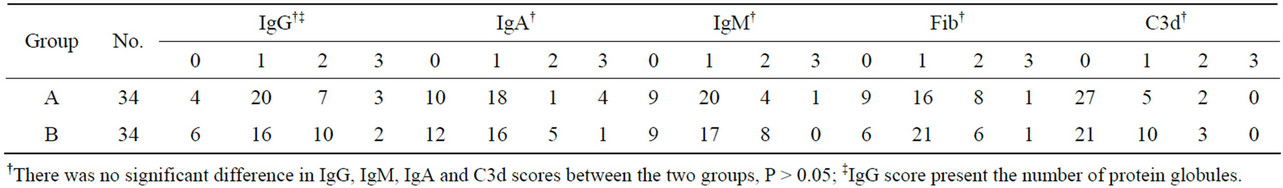
Table 2. Immunohistochemical staining results of protein globules for Groups A and B.
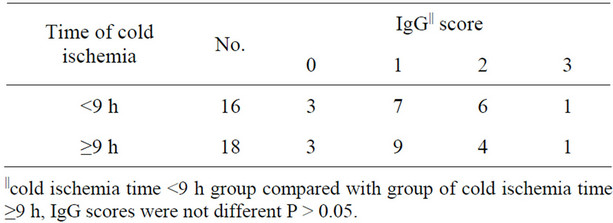
Table 4. IgG score of cases with different cold ischemia time.
cell necrosis in IRI of liver graft [3,12,13]. One form is scattered or clustered eosinophilic protein particles or apoptotic bodies, and the other is liver cell necrosis around the central vein. Both the two forms are thought to belong to the ischemic liver injury for many years [13]. In this research, we found that eosinophilic protein particles (protein globules) were stained uniformly red with PAS, which is specific staining of glycogen or polysaccharide [14]. Therefore protein globules were not fragment of RBC [15] or apoptotic bodies of liver cell, for both of which did not contain glycogen or polysaccharide [16]. The protein globules were medium electron density with no membrane-wrapping. Immunohistochemical staining showed that the protein globules contained in their plasma IgG, IgM, IgA, Fib and C3d, rather than C4d, C1q complement activation fragments [17-24], and CD61- positive platelet components [25,26], as had been previously reported in IRI and related liver cell necrosis. IgG, IgM, IgA, Fib and C3d in the protein globules may be the main components of plasma protein aggregation rather than products of a humoral immune reaction, or the microthrombi. This suggests that protein globules are not related to liver cell necrosis.
So how did protein globules form?
Table 2 shows that protein globules are present in liver graft when graft trimming and before abdomen was closed, and the number of protein globules of the two