Nephrotoxicity Evaluation in Outpatients Treated with Cisplatin-Based Chemotherapy Using a Short Hydration Method ()
1. Introduction
Oncology pharmacy is area of interest of clinical pharmacy and oncology pharmacist included in the multidisciplinary care of cancer patients [1,2]. An important role of oncology pharmacist’s is to monitor and to prevent the adverse effects of chemotherapy [3,4]. Some antineoplastic drugs associated with renal toxicity include cisplatin, carboplatin and high dose methotrexate [5]. The assessment of renal function and electrolyte levels, helps to prevent irreversible renal damage caused by nephrotoxic drugs [6,7].
Cisplatin is a platin-based antineoplastic agent [8] that also has immunosuppressive, radiosensitive, and antimicrobial properties [9]. It is used for the therapy of solid tumors, such as testicular tumors, advanced ovarian cancer, cancers of the bladder, cervix, and esophagus, lung cancer, and osteogenic sarcomas [10]. Cisplatin is used alone and in combination with other antineoplastic agents.
Cisplatin toxicity may cause ototoxic effects, peripheral neuropathy, and bone marrow suppression [10]. It’s most serious and dose-limiting adverse effect is nephrotoxicity [11-13]. Among the patients that receive a single dose (50 mg∙m–2) of cisplatin, nephrotoxicity is observed in 28% - 36%. Although this side effect is transient, depending on the dose and cumulative effect it can lead to acute tubular necrosis [9,14,15]. Moreover, it can cause glomerular damage via its negative effect on the glomerular filtration rate [11,16].
Sufficient hydration in patients before and after chemotherapy can prevent the accumulation of cisplatin in the tubules [9,10]. The method of hydration, as well as its quantity and duration vary according to the dose of cisplatin administered. In patients that receive low doses of cisplatin (25 - 35 mg∙m–2) oral hydration is adequate; however, in those that receive high doses of cisplatin
(>50 mg∙m–2) intravenous hydration and oral hydration are necessary. Intravenous administration of mannitol— an osmotic diuretic—prevents over retention of cisplatin in the kidneys [11,17].
The literature includes several studies on cisplatin nephrotoxicity, including evaluation and prevention of cisplatin-induced nephrotoxicity. Maintenance of adequate hydration is critical, especially for the prevention of nephrotoxicity; however, there is no standardization of hydration protocols [18-20]. As such, the present study aimed to evaluate cisplatin treatment protocols in outpatients with a short hydration method by monitoring renal function parameters.
2. Material and Methods
The study included 52 cancer patients undergoing cisplatin-based chemotherapy at the Marmara University Hospital, all were informed about the study, agreed to participate. The study protocol was approved by the Marmara University, School of Medicine Ethics Committee (protocol No. MAR-SBY-2007-0026). The study was conducted between February 2008 and January 2009. Data including name, address, phone number, age, gender, level of education, concomitant diseases, and treatment protocol, were obtained from patient records. In total, 3 patients dropped out of the study while undergoing chemotherapy; thus, the study was completed with the remaining 49 patients. Of the 49 patients that completed the study, 47 received chemotherapy for the first time and were treated with cisplatin alone or in combination with other agents, and 2 patients that were previously diagnosed as lung metastasis received cisplatinbased combination chemotherapy for the second time.
2.1. Cisplatin Administration
The mean quantity of cisplatin administered was 60 - 100 mg∙m–2. The cisplatin-based protocols were administered once every 21 d. Prior to cisplatin infusion, intravenous infusion of 8 mg of dexamethasone (Dekort®) and 3 mg of granisetron (Kytril®) in 150 mL of 0.9% NaCl solution for 15 min was administered as premedication to prevent nausea and vomiting. Following this procedure, cisplatin dose calculated according to the body surface area was given in 1000 mL of 0.9% NaCl solution for 90 min as an intravenous infusion. Following cisplatin infusion, 150 mL of 20% mannitol solution was administered as an intravenous infusion for 15 min.
2.2. Evaluation of Renal Functions
Blood samples were collected 1 - 3 d prior to chemotherapy and 4 - 7 d following each chemotherapy cycle [5,21-24]. All patients were monitored during 3 cycles and the 6th week of the last cycle of cisplatin chemotherapy by measuring biochemical parameters: normal ranges were 6 - 23 mg∙dL–1 for blood urea nitrogen (BUN), 0.5 - 1.1 mg∙dL–1 for creatinine (Cr), 4 - 7.0 mg∙dL–1 for uric acid (UA), 8.4 - 10.5 mg∙dL–1 for calcium (Ca), 138 - 147 mEq∙L–1 for sodium (Na), 3.5 - 5.3 mEq∙L–1 for potassium (K), 1.2 - 2.6 mg dL–1 for magnesium (Mg), and 0.5 - 0.96 mg∙L–1 for cystatin C, respectively. The patients with the history of concomitant any disease and drug use that may affect the metabolism of magnesium, potassium, sodium, and calcium were excluded from the study.
2.3. Statistical Analysis
SPSS v.11.0 was used for statistical analysis. The paired samples t test was used for renal function parameter values obtained during the 3 cycles of chemotherapy and 6 weeks after the 3rd cycle and the Wilcoxon test was used for the values that were not normally distributed. The correlation between the glomerular filtration rate (GFR) and creatinine was based on Pearson’s correlation analysis, whereas Spearman’s correlation analysis was used to determine the correlation between the GFR and cystatin C values that were not normally distributed. The level of statistical significance was set at P < 0.05.
3. Results
3.1. Patient Characteristics
Patient demographic data are given in Table 1. The mean dose of cisplatin administered during each of the 3 chemotherapy cycles was 120.30 ± 20.4 mg, 119.76 ± 24.7 mg, and 123.84 ± 16.01 mg, respectively. The total cisplatin dose administered to patients that received 2 and 3 cycles of chemotherapy was 256.87 ± 12.5 mg and 374.0 ± 39.0 mg, respectively. Table 2 shows the chemotherapy regimens administered to the patients.
3.2. Evaluation of the Renal Functions
Renal functions were monitored throughout the 3 cycles of chemotherapy. BUN, creatinine, and uric acid levels, which were normal prior to the start of chemotherapy, increased significantly (P < 0.05) during the first week of cisplatin treatment and 6 weeks after the last cycle of cisplatin-based chemotherapy; however, all three parameters remained within the limits of the reference values (Table 3).
The cystatin C levels were found above the reference values (0.5 - 0.96 mg∙L–1) in 59.5% of patients (mean of the cystatin C: 1.01 ± 0.44) after the 1st cycle, in 69.1% of patients (mean of the cystatin C: 1.07 ± 0.30) after the 2nd cycle and in 50% of patients (mean of the cystatin
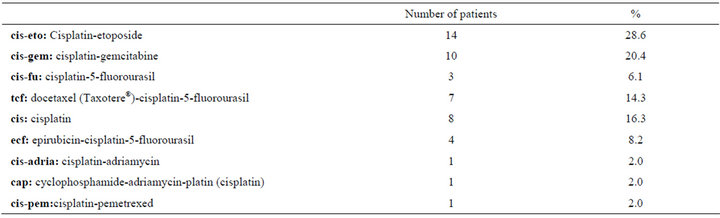
Table 2. Chemotherapy regimens administered to the patients.
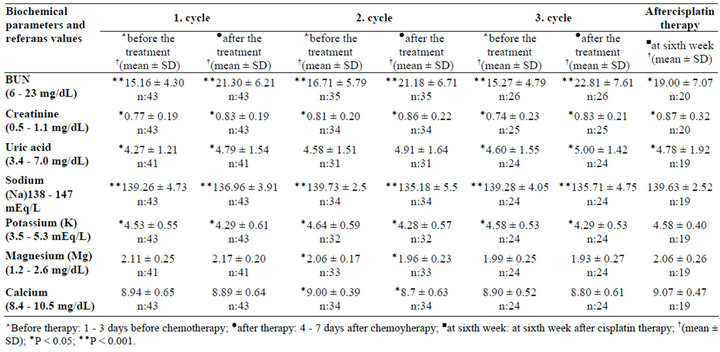
Table 3. Patients’ renal parameters.
C: 1.03 ± 0.35) of the patients after the 3rd cycle. There were also increases in cystatin C levels 6 weeks after the last cycle of cisplatin-based chemotherapy which was not statistically significant.
Patients’ electrolyte levels were evaluated during 3 cycles and the 6th week of last cycle of chemotherapy. During the 3 cycles of cisplatin-based chemotherapy decreases in potassium, magnesium, calcium and sodium levels were observed (Table 3). Sodium and potassium levels decreased significantly, whereas magnesium and calcium levels decreased only in the second cycle of chemotherapy (P < 0.05). The decreases in the Na levels were found in 56.8% of patients (mean of the Na levels: 136.96 ± 3.91 mEq∙L–1) after the 1st cycle, in 58.8% of patients (mean of the Na levels: 135.18 ± 5.5 mEq∙L–1) after the 2nd cycle and in 72% of the patients (mean of the Na levels: 135.71 ± 4.75 mEq∙L–1) after the 3rd cycle.
Estimated creatinine clearance calculated according to the Cockcroft-Gault formula [(140 – age) ´ weight/(serum creatinine ´ 72); for women this ratio was multiplied by 0.85] is expressed as the GFR. Accordingly, the GFR following the 1st, 2nd and 3rd cycles of chemotherapy were significantly lower than those before the start of chemotherapy (P < 0.01). After the 1st cycle of chemotherapy 45.5% of the patients had minimal changes in renal functions versus 32% after the 3rd cycle. The GFR 6 weeks after the end of the 3rd cycle of cisplatin chemotherapy decreased by 7%; however, the difference was not statistically significant (P > 0.05) (Table 4).
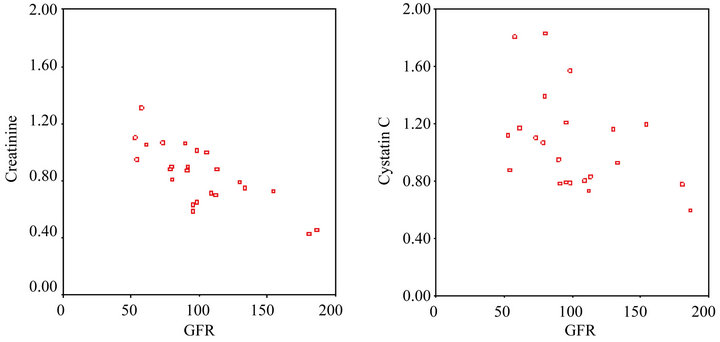
Pearson’s correlation analysis showed that prior to the start of chemotherapy there was a strong negative correlation between the GFR and creatinine level (rho: –0.701, P < 0.001), and Spearman’s correlation analysis showed that prior to the start of chemotherapy there was a strong negative correlation between the GFR and cystatin C level (rho: –0.468, P < 0.05) (Figure 1).
Pearson’s correlation analysis showed that there was a strong negative correlation between the GFR and creatinine level prior to the third cycle of chemotherapy (rho: –0.747, P < 0.001), and Spearman’s correlation analysis showed that there was a strong negative correlation between the GFR cystatin C level prior to the third cycle of chemotherapy (rho: –0.500, P < 0.05) (Figure 2).
4. Discussion
The European Society of Clinical Pharmacy (ESCP) Special Interest Group on Cancer Care published guidelines for the prevention of cisplatin nephrotoxicity in 2008 [25]. In consideration of these guidelines, patients in our study were monitored 2 - 3 d prior to the start of each chemotherapy cycle and 4 - 7 d following each cycle for serum creatinine, BUN, and uric acid levels. These parameters were statistically increased (P < 0.05);

Table 4. The GFR before and after the 1st and 3rd cycles of chemotherapy.
 (a) (b)
(a) (b)
Figure 1. (a) The correlation between the creatinine level and GFR prior to the first cycle of chemotherapy; (b) The correlation between the cystatin C level and GFR prior to the first cycle of chemotherapy.
 (a) (b)
(a) (b)
Figure 2. (a) The creatinine level and GFR following the third cycle of chemotherapy; (b) The cystatin C level and GFR following the third cycle of chemotherapy.
however, remained within the limits of the reference values.
In the literature there are many studies to evaluate the different hydration methods. A retrospective study that included patients who received 75 mg∙m–2 of cisplatin and hydration with 2 L of fluid reported that the method of hydration used was efficacious [20]. Another study reported that hydration with isotonic solution and isotonic solution plus furosemide resulted in less cisplatin-induced nephrotoxicity than compared to hydration with isotonic solution plus mannitol [19]. In the present study cisplatin was infused for 90 min in 1000 mL of isotonic solution. Following infusion, 30 mg of mannitol was infused for 15 min in 150 cc of isotonic solution. The GFR level in our patients decreased significantly after the first cycle of cisplatin-based chemotherapy (GFR: 109.62 ± 5.12 vs 99.99 ± 4.7 n = 43; P < 0.01). Again, after the third cycle of chemotherapy, the GFR decreased significantly, as compared to the pre chemotherapy value (GFR: 118.63 ± 9.0 vs 104.90 ± 8.0; n = 25; P < 0.01). The GFR levels 6 weeks after completion of cisplatinbased chemotherapy was significantly lower than the pretreatment value (GFR: 111.58 ± 9.28 vs 86.59 ± 8.20; P < 0.05). Despite the observed change, the GFR remained within normal limits and did not negatively affect the patients’ clinical presentation.
Hypomagnesemia and hyperglycaemia due to cisplatin-induced renal toxicity are common clinical conditions. Nicholas et al. reported that 87% of patients that underwent cisplatin-based chemotherapy had hypomagnesemia [26]. Another study compared oral and intravenous magnesium prophylaxis among patients treated with cisplatin that were divided into 3 groups. Group 1 was not given magnesium supplementation, Group 2 received intravenous magnesium, and Group 3 received oral magnesium supplementation. Patients were monitored throughout 4 cycles of chemotherapy and their magnesium levels were recorded and evaluated. The hypomagnesemia was observed in 33% of Group 2, 44% of Group 3, and 90% of Group 1 [27].
Our hospital’s treatment regimen does not include routine magnesium or potassium supplementation (intravenously or orally) before or after cisplatin infusion. In our study during the 3 cycles of cisplatin-based chemotherapy, sodium and potassium levels decreased significantly, whereas magnesium and calcium levels decreased only during the second cycle of chemotherapy (P < 0.05). However, potassium, magnesium and calcium levels remained within the limits of reference values. The patients’ electrolyte levels prior to the start of cisplatinbased chemotherapy and 6 weeks after completion of the therapy showed that they reverted to pre-treatment levels, indicating that the observed cisplatin-induced nephrotoxicity was transient.
The present study also monitored serum cystatin C levels and determined its correlation with the GFR. In cancer patients Stabuck et al. reported that there was a stronger correlation between creatinine clearance and cystatin C than between serum creatinine and cystatin C [28]. Many trials reported that the cystatin C level is more efficient than the serum creatinine level for evaluating cisplatin-induced decreases in the GFR [22,29,30]. The results of these studies indicate that the GFR calculated according to the Cockcroft-Gault formula and cystatin C level show a more parallel relationship than the serum creatinine level. In our study there was a strong correlation between the GFR and cystatin C level, but the correlation between the GFR and creatinine level was much stronger (cystatin C and the GFR: rho: –0.468, P < 0.05; creatinine and the GFR: rho: –0.701, P < 0.001).
In conclusion, cisplatin-induced changes in renal function using our method were transient, did not cause permanent damage to the kidneys, and did not negatively affect the patients’ clinical presentation. But it is emphasizing in the literature, when cisplatin based cycle numbers increased, depending on cumulative dose of cisplatin the changes in renal function can be permanent. Thus we suggest that patients treated with large doses of cisplatin should be monitored for nephrotoxicity. More investigations for analyzing cisplatin hydration methods used at different centers can help for standardization of hydration methods.
NOTES