Discharge heart rate and future events among Japanese patients with acute heart failure receiving beta-blocker therapy ()
1. INTRODUCTION
Evidence from clinical trials supports the use of pharmacological therapies in heart failure (HF) patients in order to improve their long-term outcome [1]. In addition to angiotensin-converting enzyme inhibitors (ACEi), aldosterone receptor antagonists, and angiotensin II receptor blockers, randomized trials have demonstrated the favorable effect of selective adrenergic receptor antagonists (beta-blockers [BBs]) such as bisoprolol [2-5], sustained-release metoprolol [6,7], and carvedilol [8-13] in HF with reduced left ventricular systolic function. As a consequence, BBs are recommended for all patients with stable HF and reduced left ventricular ejection fraction (EF) unless contraindicated [1]. Furthermore, although resting heart rate is a prognostic factor in patients with HF [14,15], the optimal heart rate in patients with HF who are receiving BBs remains unclear. Clinicians therefore attempt to titrate the dose of BB to the maximum recommended dose, and heart rate is not a focus in routine clinical settings.
In addition, ethnical variability in the use of and response to pharmacological therapies has been reported. It remains unclear how the therapeutic guidelines for HF, which are based on trials mainly conducted on Caucasian populations, can be implemented for ethnically different populations, particularly, Asian HF patients. Research on the patterns and practice outcomes in non-western countries is important since the majority of landmark studies are conducted in western countries. Furthermore, there are significant racial differences in response to BBs, and limited studies have been performed on Asian populations [16]. A study conducted in China showed large interethnic differences in the sensitivity and frequencies of genotype polymorphisms of the renin-angiotensin system [17]. In a clinical setting, Seow et al. reported high mortality in multiethnic Southeast Asian patients (67% in 5 years) despite high use of ACEi (79%), albeit with low use of BBs (5%) [18].
We sought to investigate the impact of the use of BBs on long-term outcome in patients who were admitted to a tertiary hospital for treatment of decompensated HF. In addition, we analyzed the effects of BB dose and discharge heart rate.
2. METHODS
2.1. Study Design and Patient Population
HF patients who were admitted to Keio University Hospital for HF exacerbation and successfully discharged during the period from January 2006 to August 2010 were consecutively recruited. Patients with a pacemaker (n = 25) were excluded, as were patients with no information on discharge heart rate (n = 7) or BB dose (n = 30), those who did not have follow-up data (n = 37), and those for whom the date of discharge was unknown (n = 6). From 298 recruits, the final study population comprised 199 patients. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki, and the study was approved by the institution’s human research committee.
Patients were divided into 2 groups based on the use or non-use of BBs. BB users were stratified on the basis of the dose of these medications (full dose or non-full dose). Of note, the maximum dose of BBs recommended by the Ministry of Health, Labor and Welfare in Japan is about half the dose recommended by western countries, for example, recommended dose for carvedilol 10 mg twice daily and bisoprolol 5 mg daily in Japan.
Furthermore, we divided BB users into 2 groups based on a discharge heart rate close to the average discharge heart rate of 72.6 bpm: those with a heart rate of <70 bpm and those with a heart rate of ≥70 bpm. Heart rate was measured at rest by a registered nurse before breakfast on the day of discharge. Average length of stay was 19.0 days in our cohort, which was an appropriate interval to achieve BB equilibrium.
2.2. Biomarker Measurement
CKD was defined as estimated GFR (eGFR) of <60 mL/min. Diabetes mellitus was defined according to the criteria of the American Diabetes Association. Clinical data were obtained from hospital medical charts. Plasma brain natriuretic peptide (BNP) level and other biomarkers were also measured before discharge. Commercially available assay kits were used for the measurement of BNP (Shionogi, Tokyo, Japan), cardiac troponin T (cTnT; Roche Diagnostics, Tokyo, Japan), and high-sensitivity C-reactive protein (hsCRP; Dade Behring Marburg, Tokyo, Japan). The lower limit of detection of cTnT was 0.01 ng/mL. Serum creatinine and hemoglobin levels were determined by standard laboratory methods. GFR was estimated using the equation from the Modification of Diet in Renal Disease Study: eGFR (mL·min−1·1.73·m−2) = 0.741 × 175 × Age−0.203 × SCr−1.154 (´ 0.724 for females) [19].
2.3. Follow-Up
The primary endpoint was all-cause mortality and HF requiring hospitalization. Follow-up information was obtained from medical records and inquiries directed at patients or their families via mail or telephone. Information was available for all (100%) patients. The patients were predominantly followed by cardiologists in a tertiary care setting.
2.4. Statistical Analysis
Categorical variables were expressed as numbers (percentages), and continuous variables were expressed as the mean ± standard deviation. An unpaired t-test and chi-square test were used for between-group comparison of continuous and categorical variables, respectively. Overall survival and survival without hospitalization for HF were analyzed by the Kaplan-Meier method, and the curves were compared by the log-rank test. Multivariate Cox regression analyses were performed to assess the impact of medication on outcome. The multivariate model included categorical variables that were statistically significant according to a univariate analysis as well as clinically important. A P-value of <0.05 was considered significant. Statistical analyses were performed with PASW Statistics 18.
3. RESULTS
3.1. Baseline Characteristics
A total of 199 patients were analyzed for this study, and 158 (79.4%) were on BBs at discharge. Most patients were on carvedilol (n = 152), and a few were on bisoprolol or atenolol (n = 3 respectively). The mean age was 66.8 years; 70.3% of patients were men. The prevalence of ischemic cardiomyopathy, diabetes mellitus, and CKD was 30.2%, 32.2%, and 66.8%, respectively. A total of 158 (79.4%) patients were receiving renin-angiotensin system inhibitors (RASi).
The mean hemoglobin level was 13.0 g/dl. The median follow-up period for this cohort was 1.5 years (interquartile range, 0.6 - 2.6 years).
Compared to non-users of BBs, BB users were younger (65.0 ± 15.5 vs 73.8 ± 13.3 years, P = 0.001) and had a lower EF (36.0% ± 14.0% vs 48.3% ± 11.7%, P < 0.001). The prevalence of ischemic cardiomyopathy was also higher among BB users (34.2% vs 14.6%, P = 0.015) (Table 1). The primary endpoint was observed in 58 (36.7%) BB users and in 13 (31.7%) BB non-users (logrank test P = 0.860) (Figure 1). Cox proportional hazard analysis revealed that BB use had no influence on long-term outcome after adjustment for baseline EF and age, CKD, creatinine, BNP, RASi use, and potassium, which were identified as significant in the univariate analysis (HR = 2.564, P = 0.246).
3.2. Dose of Beta-Blockers
Of the 158 BB users, 19 and 139 were receiving a full dose and a non-full dose, respectively. The BNP value was lower among patients who were receiving the full dose of BB (220.6 ± 148.9 vs 368.4 ± 445.0, P = 0.007) (Table 2). The primary endpoint was observed in 8 (42.1%) patients receiving the full dose and in 50 (36.0%) patients receiving a non-full dose of a BB (log-rank testTable 1. Baseline characteristics by use of BB.

Values are expressed as mean ± SD or n (%). P refers to comparison between patients who were on BB and those who were not on BB. BB, beta blocker; BNP, brain natriuretic peptide; CRP, C-reactive protein; Hb, hemoglobin; NYHA, New York Heart Association; RASi, renin-angiotensin system inhibitor.
Table 2. Baseline characteristics by dose of BB.

Values are expressed as mean ± SD or n (%). BB, beta blocker; BNP, brain natriuretic peptide; CRP, C-reactive protein; Hb, hemoglobin; NYHA, New York Heart Association; RASi, renin-angiotensin system inhibitor.
P = 0.701) (Figure 2). Cox proportional hazard analysis revealed that the BB dose had no influence on long-term outcome after adjustment for age, gender, and baseline EF (HR 1.173, P = 0.680).
3.3. Beta-Blockers and Heart Rate
Of the 158 BB users, 61 had a discharge heart rate of <70 bpm. There were no significant differences between the 2 groups in terms of age (P = 0.770), prevalence of diabetes mellitus (P = 0.392), CKD (P = 0.837), use of RASi such as ACEi (P = 0.282), and BNP (P = 0.300) (Table 3). The primary endpoint was observed in 16 (26.2%) and 42 (43.4%) patients with a discharge heart rate of <70 bpm and ≥70 bpm, respectively (log-rank P = 0.05) (Figure 3). Discharge heart rate was a significant predictor of long-term outcome after adjustment for age, gender, and baseline EF (HR 1.872; P = 0.040). Furthermore, discharge heart rate was not correlated with BB dose at discharge among BB users (Spearman Rank Correlation Coefficient = −0.012, P = 0.858).
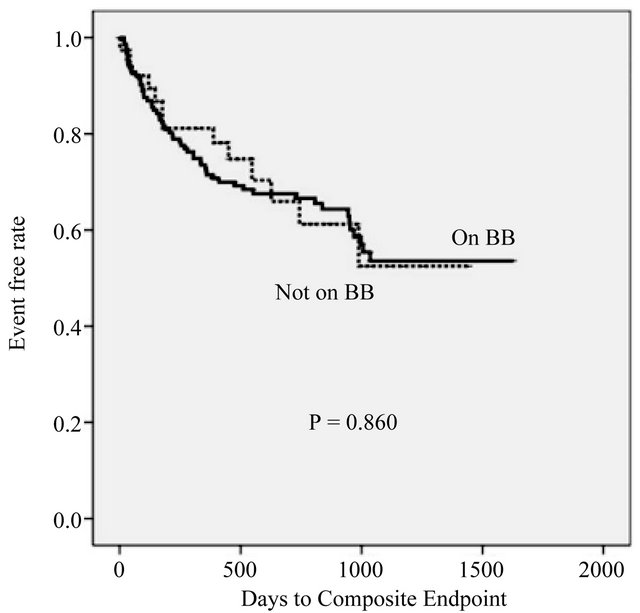
Figure 1. Kaplan-Meier event curve for on vs not on BB group.

Figure 2. Kaplan-Meier event curve for full dose vs not-full dose group.
4. DISCUSSION
The main findings of the present study were as follows: 1) BB prescriptions based on physician preference had a limited impact on outcome in a cohort of Japanese HF patients who had recovered from recent acute decompensation; 2) full dose of BBs was not associated with improved outcome; 3) optimal heart rate appeared to reduce the risk of a HF event in BB users.
European and American guidelines for the treatment of HF have reached a consensus by incorporating numerous published studies and knowledge into clinical practice. [20,21] The use of RASi, diuretics, and BBs in the ma-

Figure 3. Kaplan-Meier event curve for HR > 70 vs HR ≤ 70 group.
jority of HF patients was already recommended at the time of our study [20,21]. Overall, adherence to evidence-based medication in the present study was consistent with that in western countries; 79.4% of the patients received RASi at discharge, which is comparable to the approximately 60% in Europe [22,23] and 88% in the United States [24]. Similarly, 79.4% of our patients were discharged with a BB; this percentage was 83.5% in the US [25]. Traditionally, the number of clinical research studies performed in Japan has been limited, and prescription of guideline-based medication has been low compared to that in western countries. For example, in a multicenter, prospective cohort study conducted in Japan between 2000 and 2005, only 29.9% of patients with chronic HF were using a BB [26]. In another Japanese registry study performed between 2004 and 2005, 65.9% of patients with systolic dysfunction were using a BB at the time of hospital discharge. The majority of patients taking a BB received carvedilol (89.8%) [27]. Although our study was limited to a single academic institution, we believe that improvement of the BB prescription rate and RASi use represents strong implementation of evidencebased therapy in Japan.
Resting heart rate is a prognostic factor in patients with HF [14,15], but the optimal heart rate in patients with HF who are using BBs remains unclear. Therefore, clinicians attempt to titrate the dose of BB to the maximum recommended dose, and heart rate is not a focus in routine clinical settings. In the present study, a discharge heart rate of <70 bpm was associated with improved outcome in BB users. In the SHIFT study, a direct association was found between baseline heart rate and
Table 3. Baseline characteristics of patients discharged on beta-blocker, stratified by discharge heart rate.

Values are expressed as mean ± SD or n (%). BB, beta blocker; BNP, brain natriuretic peptide; CRP, C-reactive protein; Hb, hemoglobin; NYHA, New York Heart Association; RASi, renin-angiotensin system inhibitor.
outcomes in patients with HF who were in sinus rhythm with a heart rate of ≥70 bpm [28]. Moreover, in a metaanalysis of BB trials on HF conducted in western countries, the benefit on mortality was related to the reduction in heart rate achieved in each trial, and was not related to the BB dose [29].
In the present study, patients who were receiving a full dose of a BB did not have an improved outcome compared to those who were not receiving a full dose. There is little information on the response to BBs among Japanese patients. In 1 small study, carvedilol reduced hospitalization for cardiovascular disease in a dose-dependent fashion, and even a low dose of carvedilol (as low as 5 mg/day) was effective [30].
In controlled clinical trials, the BB dose is not determined by a patient’s therapeutic response, but is increased until the patient has received a prespecified target dose. Low doses are prescribed only if the target doses are not tolerated; therefore, most trials have not evaluated whether low doses are effective. The implication is that physicians, especially cardiologists and primary care physicians, should make every effort to achieve the target doses of the BB shown to be effective in major clinical trials [1]. Our results challenge the above notion and indicate that optimizing heart rate, rather than maximizing dose, is an appropriate treatment strategy for the betasensitive Japanese population.
Despite aggressive use of BBs in our population, the impact of BBs might have been diluted by the physician’s preference for its use. One study reported that more than a quarter of users discontinued BBs in the first year after hospitalization and that the 5-year compliance rate was 53% [31]. In a study conducted in the US, withdrawal of BBs was associated with a substantially higher adjusted risk of mortality compared with BB continuation, but with a similar risk to that of eligible HF patients not treated with BBs. Furthermore, users of BBs had a lower EF, which can contribute to higher mortality. Multivariate regression analysis of covariates such as age, CKD, creatinine, BNP, RASi use, and potassium, which were shown to be significant in univariate analysis, revealed no significant differences between the 2 groups (users and non-users of BBs; HR = 2.914, P = 0.16). However, the applicability of these results may be limited by the low number of patients included in our analysis.
There are several limitations to the current study. Although the results remained significant, we were unable to adjust for significant variables in the subgroup analysis since the number of patients included in this study was relatively small. Further, adherence to evidencebased medication may have impacted the outcome. In our cohort, the BB dose remained the same in 53.2% of the patients during the follow-up period, but due to the limited number of patients and the short follow-up period, we did not perform any additional analyses. We did not classify patients based on systolic and diastolic HF, although not all HF patients have a similar response and outcome from this perspective. Lastly, use of spironolactone in symptomatic HF patients with systolic left ventricular dysfunction was not included in our analysis. Various comorbidities, such as renal insufficiency or hypotension, may have contributed to non-adherence to evidence-based medication. However, without rigid prospective definitions, it is not possible to determine whether other solutions could be found without discontinuing these agents.
In conclusion, BB therapy was not associated with better long-term outcomes in this cohort. Our results suggest that in BB therapy, optimizing heart rate is more important than dose titration.
5. ACKNOWLEDGEMENTS
We wish to acknowledge the patients involved in the cohort.

