1. Introduction
Copper is an essential element not only to life in mammals but also to plants and lower forms of organisms. Copper is an essential trace mineral barely does justice when one considers the wide range of vital human bodily functions dependent upon this mighty mineral. To begin with, copper is a major component of hemoglobin, the protein responsible for oxygen transport in blood cells. Copper, along with vitamin C, forms elastin, a protein required to keep skin, blood vessels, and lungs supple and elastic. As an antioxidant, copper plays a strong dual role. First as a central component of both the superoxide dismutase molecule, which protects us from cellular freeradical damage. Secondly, copper helps form the protein ceruloplasmin, which protects us against free-radical damage caused by iron. Copper is also required by the central nervous system as a component in the production of noradrenaline, the brain’s version of adrenaline and the neurotransmitter that keeps us alert. Copper is also involved in the production of prostaglandins, hormone like chemicals that regulate blood pressure, pulse, and healing. It has various and many biologic effects as an essential element and also as a toxic one. It is usually used as an algaecide and herbicide. Copper can be toxic to aquatic plants and some fish at concentrations less than 1 g·m–1. Thus, copper tends to be much more of an environmental hazard than a human hazard. Most environmental, biological and alloy samples generally have a trace amount of copper at level of n g·mL–1. [1-3]
Generally determination of trace metal is very important in the context of environmental protection, food and agricultural chemistry and high purity materials. Flame atomic absorption spectrometry and spectrophotometeric have been widely used for the determination of trace metal ions because of the relativity simple and inexpensive equipment required. The concept of extraction and preconcentration play important roles in many analytical procedures. Solvent extraction and solid phase extraction are arguably the most commonly executed forms of preconcentration and for many years they have dominated approaches to the enrichment of pesticides, drugs and trace metals. There are, however, physical difficulties associated with the use of solvent extraction for the enrichment of large numbers of samples and/or enrichment from large sample volumes. Solvent extraction requires vigorous agitation, to ensure complete partition of the analyte between two immiscible phases and this can only be achieved by the application of significant human or mechanical effort. In addition, there are increasing environmental and cost pressures to replace, or at the very least reduce, the volumes of solvents employed in analytical procedures. Many adsorption materials such as organic chelate resin, silica gel, activated carbon, activated alumina, zeolites and microcrystalline materials are commonly used as adsorbents [4-8]. Nowadays, and nanometer materials have become more important owing to its special physical and chemical properties. The field of nanocomposite materials has received the attention, imagination and close scrutiny of scientists and engineer in recent years. These particles fall within the colloidal range, exhibiting typical colloidal properties. One of the specific properties of nanomaterials is that a high percent of atoms of the nanoparticle is on the surface. The surface atoms are unsaturated and can therefore bind with other atoms, possess high chemical activity. Nanoparticles exhibit intrinsic surface reactivity and high surface areas and can strongly chemisorb many substances. The size, surface structure and interparticle interaction of nanomaterials determine their unique properties and the improved performances and make their potential application in many areas [8,9]. In present work, chemically grafted SiO2-RATP nanoparticles have been used for the preconcentration and separation of copper prior to their determination by spectrophotometric method [10].
2. Experimental
2.1. Apparatus
Absorbance of Cu(II) was measured with UV-Vis Shimadzu-1700 spectrophotometer. The pH values were controlled by century Cp-901 digital pH meter.
2.2. Reagents and Standard Solutions
Unless otherwise stated, all reagents used were of analytical reagent grade and all solutions were prepared with double distilled deionized water. The 3-aminopropyl-triethoxysilane of GR grade was supplied by Acros Organics (USA). Resacetophenone was obtained from Merck (Mumbai). Nanometer SiO2 was synthesized according to the method reported [11]. The glassware was washed with chromic acid and soaked in 5% nitric acid overnight and then cleaned with double distilled water before use.
2.3. Modification Process
Surface modification of SiO2 nanoparticles were performed in a 250 mL flask. Nanometer SiO2 (1 g) was dispersed into dry toluene (30 mL), and then 3-aminopropyltriethoxysilane (4 mL) was gradually added, with continuous stirring. The mixture was refluxed for 6 h. The silylated nanometer SiO2 was filtered off, washed with toluene and ethanol and dried at 60˚C for 3 h. The product was transferred into the flask, and then 30 mL diethyl ether was added followed by 2 g of RATP and refluxed at 72˚C for 4 h. Reaction mixture was filtered under vaccum.
2.4. General Procedure
Aliquots of sample solutions containing the analytes of interest were prepared and pH was adjusted to the desired value with boric acid/borax buffer. Then, 25 mg of SiO2- RATP nanoparticles were added, and the mixture was shaken vigorously for 11 mins to facilitate adsorption of metal ion onto the adsorbent. Cu(II) retained on the adsorbent was eluted with 4 M (5 mL) hydrochloric acid, and the elution was neutralized with 2M sodium hydroxide. Then, these metal ions were filtered and were determined by standard spectrophotometric method [14].
3. Result and Discussion
3.1. Characterization of SiO2-RATP Nanoparticle
The modification of nanometer-sized material is usually required in order to prevent a conglomeration of particles and to improve its consistency in relation to other materials, such as organic polymers. In addition, for the purpose of separation, the modification of nanometer-sized materials can improve the selectivity of nanometer-sized materials towards metal ions, organosiloxane is the most often used modifiers. The modified nanometer SiO2-RATP was characterized SEM.
3.2. Scanning Electron Microscopy
The average diameter of the nanoparticles SiO2, SiO2- APTES and SiO2-RATP was 100 nm, 300 nm and 1 μm confirmed by Scanning Electron Microscopy. Figures 1-3 reveals the average size of SiO2 nanoparticle, SiO2- APTES and SiO2-RATP respectively.
3.3. Effect of pH on Enrichment Recovery
The adsorption of Cu(II) on nanometer SiO2-RATP was studied at different pH value (3.4 to 10.0) following the recommended procedure. The results of effect of pH on the recoveries of the metal ions were shown in Figure 4. It can be seen that a quantitative recovery (≥95%) was found for Cu(II) in the pH range of 7 - 10.

Figure 1. Scanning electron micrograph of SiO2 nanoparticles.
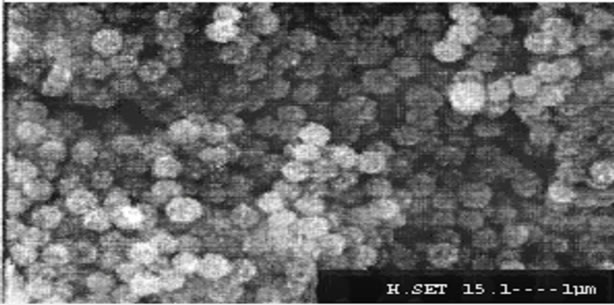
Figure 2. Scanning electron micrograph of SiO2-APTES nanoparticles.
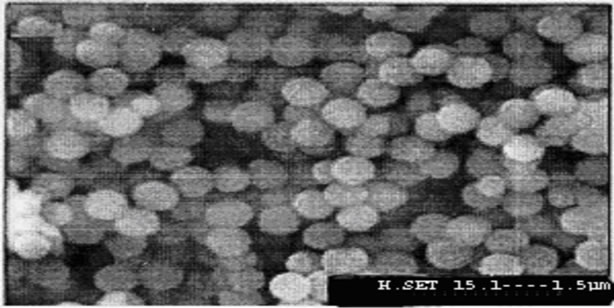
Figure 3. Scanning electron micrograph of SiO2-RATP nanoparticles.

Figure 4. Effect of pH on the recovery of analyte.
3.4. Effect of Eluent Concentration and Volume
Elution of Cu(II) from nanometer SiO2-RATP extractant was investigated by using various concentrations of hydrochloric acid. It can be seen that quantitative recoveries (≥95%) of Cu(II) can be obtained using 5 mL of 4 M hydrochloric acid as eluent. Therefore, 5 mL of 4 M was used as eluent in subsequent experiments. The results of effect of eluent concentration and volume are given in Tables 1 and 2.

Table 1. Effect of concentration of HCl solution on elution of Cu(II) (n = 3).

Table 2. Effect of volume of HCl solution on elution of Cu(II) (n = 3).
3.5. Effect of Nanometer SiO2-RATP Amount
To test the effect of amount of extractant on quantitative retention of analyte, different amount (10 - 50 mg) of nanometer SiO2-RATP were added into the solution following the experimental method. 25 mg of nanometer SiO2-RATP as extractor was found to be sufficient for further studies. The results are shown in Figure 5.
3.6. Effect of Shaking Time
The adsorption of Cu(II) on 25 mg of nanometer SiO2- RATP was studied for different shaking time (5 - 35 mins). The results indicated that within 15 mins the extraction percentage of Cu(II) ≥ 95% was achieved. The results are shown in Figure 6.
3.7. Adsorption Capacity (QS)
The adsorption capacity is an important factor as it determines how much adsorbent is quantitatively required to concentrate the analytes from a given solution. A breakthrough curve was obtained by plotting the concen-

Figure 5. Effect of sorbent amount on the recovery of analyte.

Figure 6. Effect of shaking time on the recovery of analyte.
tration (mg/L) vs. the mmol of Cu(II) adsorbed per gram. From the breakthrough curve the amount of modified nanometer SiO2-RATP for Cu(II) was found to be 61.50 µmol/g at pH 8.0. The results are shown in Figure 7.
3.8. Effect of Sample Volume
In order to explore the possibility of concentrating low concentration of analytes from large volumes, the effect of sample volume on the retention of metal ions was also investigated. For this purpose 50, 70,100, 150, 200, 250, 300, 350 and 400 mL of the sample solutions containing 1.0 µg Cu(II) was shaken, quantitative recoveries (≥95%) were obtained for sample volume of ≤ 300 mL for Cu(II). Therefore, 50 mL of sample volume solution was adopted for the preconcentration of analytes from sample solutions. The results are given in Figure 8.
3.9. Effect of Coexisting Ions
The effect of common coexisting ions on the sorption of Cu(II) was investigated. In these experiments, a solution of 1.0 µg/mL of Cu(II) that contains the added interfering ion was analyzed according to the recommended procedure. The tolerance limit (mg/mL) for anions such Cl–1, Br–1,  ,
,  ,
,  and EDTA The tolerance limits of these anions in mg·ml–1 were 0.20, 0.26, 0.04, 0.15, 0.05 and 1.0 respectively. The tolerance limits in mg/mL for Ca(II), Mg(II), Fe(II), Co(II), Ni(II), Cr(III), Pb(II), Mn(II), Zn(II) and Cd(II) were 0.20, 0.05, 0.11, 0.010, 0.012, 0.012, 0.09, 0.06, 0.011 and 0.09 respectively.
and EDTA The tolerance limits of these anions in mg·ml–1 were 0.20, 0.26, 0.04, 0.15, 0.05 and 1.0 respectively. The tolerance limits in mg/mL for Ca(II), Mg(II), Fe(II), Co(II), Ni(II), Cr(III), Pb(II), Mn(II), Zn(II) and Cd(II) were 0.20, 0.05, 0.11, 0.010, 0.012, 0.012, 0.09, 0.06, 0.011 and 0.09 respectively.

Figure 7. Adsorption capacity of Cu(II) on nanometer SiO2-RATP.

Figure 8. Effect of volume of sample solution on the recovery of analyte.
4. Analytical Precision and Detection Limit
Under the selected conditions, three portions of Cu(II) standard solutions was enriched and analyzed following the general method. The relative standard deviation (RSD) of the method was 2.4%. The detection limit of this method for Cu(II) was 0.36 µg·L–1.
5. Applications
The developed method has been applied for the determination of trace Cu(II) in natural water samples. The results are given in Table 3.
Sample Preparation
Rice flour, corn flour and black tea were obtained from supermarkets of Patiala, Punjab-India. An amount of 0.2 g of dried sample was transferred to a Teflon vessel and 2 mL of HNO3 65% and 2 mL of deionized water were added. The digestor was heated for 4 h at 100˚C. After cooling at room temperature the solutions were adjusted

Table 4. Copper determination in food samples (n = 3).

Table 5. Figure of merit of comparable methods for the determination of Hg(II) by solid-phase extraction.

Table 6. Comparison of Limit of detection using SiO2-PAN nanoparticles with other sorbents.
to pH with a 10% sodium hydroxide solution. Results are given in Table 4.
6. Conclusions
The preconcentration method described by using Resacetophenone anchored silica nanoparticles for the determination of Cu(II) in water samples has a good accuracy, repeatability and sensitivity. The preparation of sorbent is easy and the preconcentration factors obtained are sufficiently large. The results of comparison of preconcentration factor and limit of detection were given in Tables 5 and 6.