Sulfuric Acid Immobilized on Silica Gel as Highly Efficient and Heterogeneous Catalyst for the One-Pot Synthesis of 2,4,5-Triaryl-1H-imidazoles ()
1. Introduction
Multicomponent reactions (MCRs) have drawn great interest enjoying an outstanding status in modern organic synthesis and medicinal chemistry because they are onepot processes bringing together three or more components and show high atom economy and high selectivity [1,2].
MCRs have great contribution in convergent synthesis of complex and important organic molecules from simple and readily available starting materials, and have emerged as powerful tools for drug discovery [3,4]. The imidazole nucleus is a fertile source of biologically important molecules. Compounds containing imidazole moiety have many pharmacological properties and play important roles in biochemical processes. They are well known as inhibitors of P38MAP kinase, fungicides, herbicides, antiinflammatory agents, antithrombotic agents, plant growth regulators and therapeutic agents. In addition, they are used in photography as photosensitive compounds. Some substituted triarylimidazoles are selective antagonists of the glucagons receptor and inhibitors of IL-1 biosynthesis [5]. Radziszewski and Jaap proposed the first synthesis of the imidazole core in 1882, starting from 1,2-dicarbonyl compounds, aldehydes and ammonia to obtain 2,4,5-triphenylimidazole [6,7]. There are several methods for the synthesis of 2,4,5-triarylimidazoles using H3PO4·12MoO3·24H2O, KH2PO4 [8], catalyst-free under microwave irradiation [9,10], ionic liquid (1-n-butyl and 1,3-di-butyl imidazolium salts) [11], ceric (IV) ammonium nitrate (CAN) [12], oxalic acid [13], Eu(OTf)3 [14], [Hmim]HSO4 [15], ZrCl4 [16], Yb(OTf)3 [17], NiCl2·6H2O [18], sodium bisulfate [19], iodine [20], nanocrystalline magnesium Oxide [21], oxalic acid [22], silica sulfuric acid [23], acetic acid [24], L-proline [25], PEG-400 [26], Cu(TFA)2 [27], tetrabutylammoniumbromide (TBAB) [28], (NH4)6Mo7O24·4H2O [29], InCl3·6H2O [30], Zr(acac)4 [31], anhydrous FePO4 [32] and uranyl nitrate hexahydrate [UO2(NO3)2·6H2O] supported on acidic alumina [33].
Many of these methods, however, suffer from longer reaction times, unsatisfactory yields, difficult workup, and excessive use of reagents and catalyst. It is therefore important to find more convenient methods for the preparation of these compounds. Therefore, the development of a new mild method to overcome these disadvantages still remains a challenge for organic chemists. One of the aims we have in mind is to introduce a new catalyst for synthesis of 2,4,5-trisubstituted imidazoles with cost effectiveness and mild condition in high yields.
2. Result and Discussion
Several methods are used in the synthesis of these trisubstituted imidazoles and their derivatives. In addition, the synthesis of these heterocycles has been usually carried out in polar organic solvents such as ethanol, methanol, acetic acid, DMF and DMSO leading to complex isolation and recovery procedures. These processes also generate waste containing catalyst and solvent, which have to be recovered, treated and disposed of. The toxicity and volatile nature of many organic solvents, particularly chlorinated hydrocarbons that are widely used in huge amounts for organic reactions have posed a serious threat to the environment [34]. Thus, design of solvent-free catalytic reaction has received tremendous attention in recent times in the area of green synthesis [35].
Solid acids and especially those based on micelletemplate silica’s and other mesoporous high surface area support materials are beginning to play a significant role in the greening of fine and specialty chemicals manufacturing processes. A wide range of important organic reactions can be efficiently catalyzed by these materials, which can be designed to provide different types of acidity as well as high degrees of reaction selectivity. The solid acids generally have high turnover numbers and can be easily separated from the organic components [36,37]. In recent years the H2SO4 immobilized on SiO2 was used as a catalyst for synthesis of organic compounds [38-42]. In this work, we report the solvent-free synthesis of 2,4,5-trisubstituted imidazoles using H2SO4 immobilized on SiO2 as a catalyst under classical heating (Scheme 1).
Efficiency of this reaction is mainly affected by the amount of catalyst, temperature and reaction time. For getting the best conditions, initially we started the condensation of benzil (1 mmol), 4-chloro benzaldehyde (1 mmol) and ammonium acetate (5 mmol) in the presence of H2SO4 immobilized on SiO2 (0.005 gr) as a catalyst at 100˚C for 1 h, which led to low yield (40%) of 2,4, 5-trisubstituted imidazole (Table 1, entry 1). To enhance the yield of the desired product the temperature of the reaction was increased to 120˚C (entry 2, 3). With increasing the temperature, the productivity of the reaction increased but was not very high. Then, it was thought worthwhile to carry out the reaction in the presence of higher amount of the catalyst (entry 4, 5). As indicated in Table 1, Maximum yield was obtained (94%) when the reaction was loaded with 0.01 gr of the catalyst at the 110˚C. A further increasing of catalyst loading does not affect the yield (entry 6).
After optimizing the conditions, we applied this catalyst for synthesis of trisubstituted imidazoles by using different aromatic aldehydes with a wide range of ortho-, metaand para-substitutions under solvent-free classical heating conditions to establish the catalytic importance of H2SO4 immobilized on SiO2 for this reaction.
Generally, the synthetic procedure involves stirring the mixture of aldehyde (1 mmol), benzil (1 mmol), ammonium acetate (5 mmol) and H2SO4 immobilized on SiO2 (0.01 gr) for 45 - 60 min at 110˚C. The corresponding results are given in Table 2. We found that the reaction proceeded very efficiently either electron-releasing or electron-withdrawing substituents on aryl ring of aldehyde.
Also, due to direct use of benzoin rather than benzil in the synthesis of imidazoles a significant improvement in the synthesis toward the greener chemistry is represented. We have repeated the reaction with benzoin instead of benzil and the desired product has been efficiently produced. As indicated in Table 2, when we used benzoin instead of benzil, the reaction time increased and also the yield of the reaction decreased partially.
Possible mechanism for the sulfuric acid immobilized on silica gel catalysed synthesis of trisubstituted imidazoles has been proposed in Scheme 2.
In summary, this paper describes a convenient and efficient process for the Solvent-free synthesis of trisubstituted imidazoles through the three-components coupling of benzil or benzoin, aldehydes and ammonium acetate using H2SO4 immobilized on SiO2 as a catalyst. Reaction profile is very clean and no side products are formed. All the synthesized imidazoles have been characterized on the basis of elemental and spectral studies. We believe that this procedure is convenient, economic, and a userfriendly process for the synthesis of trisubstituted imidazoles of biological and medicinal importance.
Also, we investigated the reusability and recycling effect of H2SO4·SiO2 catalyst in these reactions. At the end of each reaction, the catalyst was filtered, washed with diethyl ether, dried at 120˚C for 3 h, and reused in a subsequent reaction cycle. The recycled catalyst was employed consecutively for three reactions and no significant loss in its efficiency was observed (Table 2. 2a, 2d,
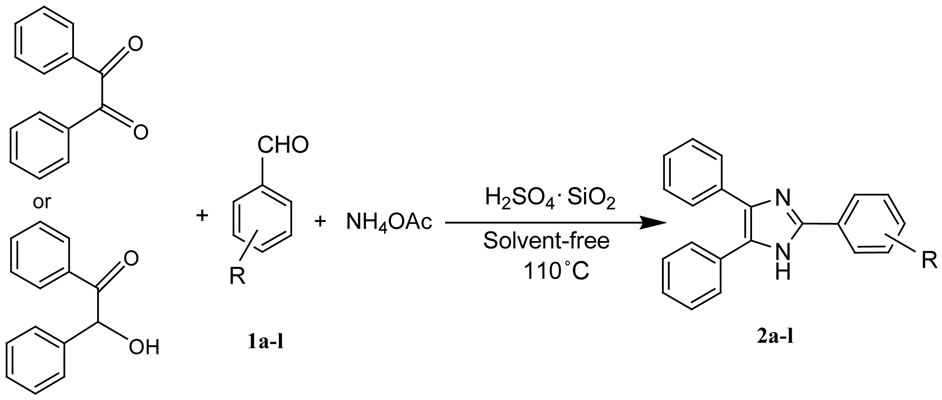
Scheme 1. Sulfuric acid immobilized on silica gel catalysed synthesis of 2,4,5-trisubstituted imidazole.

Scheme 2. Probable mechanism for the formation of triarylimidazoles using benzil or benzoin, ammonium acetate, aromatic aldehydes using sulfuric acid immobilized on silica gel as catalyst.

Table 1. Optimization one-pot synthesis of trisubstituted imidazoles under classical heating conditionsa.


Table 2. Synthesis of 2,4,5-triaryl-1H-imidazoles (2a-l) using H2SO4·SiO2 (0.01 gr) under solvent-free conditions.
2i and 2k).
3. Experimental
3.1. Instruments and Characterization
Melting points were measured with an Electrothermal 9100 apparatus. IR spectra were recorded with a Varian 3100 FTIR spectrometer. CHN analyses were performed on Exeter Analytical Inc. “Model CE-400 CHN Analyzer”. 1H and 13C NMR spectra were recorded with a BRUKER DRX-400 AVANCE spectrometer at 298˚K and 75.47 MHz, respectively. NMR spectra were obtained on solutions in DMSO-d6. All the products are known compounds, which were characterized by IR and 1H NMR spectral data and their melting points were compared with literature reports.
3.2. Preparation of the H2SO4·SiO2 Catalyst
To a slurry of silica gel (10 g, 200 - 400 mesh) in dry diethyl ether (50 ml) was added concentrated H2SO4 (3 ml) with shaking for 5 min. The solvent was evaporated under reduced pressure to obtain dry H2SO4·SiO2 catalyst which was then heated at 120˚C for 3 h.
3.3. General Procedure for Preparation of 2a-l
A mixture of aldehyde (1 mmol), benzil or benzoin (1 mmol), ammonium acetate (5 mmol) and H2SO4·SiO2 (0.01 gr), as a catalyst, in a 20 ml glass tube was stirred at 110˚C for 45 - 75 min. After completion of the reaction, the reaction was cooled to room temperature and solid materials were washed with water and the solvent was evaporated to give the crude product. For further purification it was recrystallized from ethanol 96% to afford pure product.
3.4. The Spectral Data for Selected Compound
2,4,5-Triphenyl-1H-imidazole (2a):
Mp 273˚C - 275˚C. FTIR (KBr, cm–1): 3451, 2856, 1636, 1490. 1H NMR (400 MHz, DMSO-d6): δ = 12.69 (s, 1H), 8.09 (d, 2H), 7.56 - 7.22 (m, 13H). 13C NMR (75 MHz, DMSO-d6) δ: 145.6, 137.2, 135.2, 131.2, 130.4, 128.7, 128.5, 128.3, 128.2, 127.8, 127.2, 126.6, 125.3.
2-(4-Chlorophenyl)-4,5-diphenyl-1H-imidazole (2b):
Mp 264˚C - 266˚C. FTIR (KBr, cm–1): 3452, 3065, 1635, 1323. 1H NMR (400 MHz, DMSO-d6): δ = 12.78 (s, 1H), 8.11 (d, 2H), 7.56 - 7.23 (m, 12H). 13C NMR (75 MHz, DMSO-d6) δ: 146.3, 130.3, 129.9, 129.2, 128.5, 127.4, 127.0, 126.4, 125.5, 125.2, 123.3, 116.3.
4,5-Diphenyl-2-p-tolyl-1H-imidazole (2c):
Mp 231˚C - 232˚C. FTIR (KBr, cm–1): 3449, 3034, 1611, 1495, 1320. 1H NMR (400 MHz, DMSO-d6): δ = 12.59 (s, 1H), 7.98 (d, 2H), 7.54 - 2.21 (m, 12H), 2.35 (s, 3H). 13C NMR (75 MHz, DMSO-d6) δ: 145.6, 137.6, 136.9, 135.2, 131.1, 129.2, 128.6, 128.3, 128.1, 127.9, 127.6, 127.0, 126.4, 125.1, 20.8.
2-(4-Methoxyphenyl)-4,5-diphenyl-1H-imidazole (2d):
Mp 230˚C - 233˚C. FTIR (KBr, cm–1 ): 3425, 3029, 2956, 1610, 1495, 1249. 1HNMR (400 MHz, DMSO-d6): δ = 12.50 (s, 1H), 8.03 (d, 2H), 7.50 - 7.33 (m, 10H), 7.05 (d, 2H), 3.82 (s, 3H). 13C NMR (75MHz, DMSO-d6) δ: 159.5, 145.7, 128.4, 127.7, 126.8, 123.1, 114.1, 55.2.
2-(4-Fluorophenyl)-4,5-diphenyl-1H-imidazole (2f):
Mp 250˚C - 252˚C. FTIR (KBr, cm–1): 3316, 2993, 2470, 1660, 1210, 1169, 874, 719, 639. 1H NMR (400 MHz, DMSO-d6): δ = 12.82 (s, 1H), 8.28 (d, 2H), 7.22 - 7.55 (m, 10H), 7.03 (d, 2H). 13C NMR (75 MHz, DMSO-d6) δ: 165.4, 137.3, 131.1, 129.8, 128.9, 127.7, 127.2, 126.6, 125.9, 125.5, 124.1, 117.4.
2-(2-Methoxyphenyl)-4,5-diphenyl-1H-imidazole (2g):
Mp 212˚C - 213˚C. FTIR (KBr, cm–1): 3437, 3033, 2950, 1615, 1498. 1H NMR (400 MHz, DMSO-d6): δ = 11.82, (s, 1H), 8.02 (d, 1H), 7.53 - 7.07 (m, 13H), 3.92 (s, 3H). 13C NMR (75MHz, DMSO-d6) δ: 158.2, 146.2, 128.3, 127.5, 125.4, 123.8, 115.4, 55.3.
2-(3-Nitrophenyl)-4,5-diphenyl-1H-imidazole (2i):
Mp 301˚C - 302˚C. FTIR (KBr, cm–1): 3448, 3068, 1526, 1350. 1H NMR (400 MHz, DMSO-d6): δ = 13.10 (s, 1H), 8.95 (s, 1H), 8.53 (d, 1H), 8.23 (d, 1H), 7.81 (d, 1H), 7.54 - 7.33 (m, 10H). 13C NMR (75 MHz, DMSO-d6) δ: 148.4, 143.4, 131.8, 131.2, 130.4, 128.7, 128.4, 127.1, 122.6, 119.4.
4. Conclusion
We have been able to introduce an efficient and environmentally friendly approach for the synthesis of biologically active trisubstituted imidazoles via condensation of benzil or benzoin with various aromatic aldehydes and ammonium acetate using sulfuric acid immobilized on silica gel as a catalyst. High yields, easy work-up, purification of compounds by non-chromatographic method (crystallization only) and the reusability of the H2SO4·SiO2 catalyst are the key advantages of this method.
NOTES