Carvedilol vs. metoprolol: A comparison of effects on endothelial function and oxidative stress in response to acute hyperglycemia in patients with type 2 diabetes and hypertension ()
1. INTRODUCTION
Over 75% of patients with diabetes die from cardiovascular disease (CVD) related complications. Prevention of CVD risk factors including hyperglycemia and hypertension has been demonstrated to reduce CVD events in diabetics [1,2]. Beta blockers have been a cornerstone in the treatment of hypertension and secondary prevention of CVD and congestive heart failure [1]. However, concerns about masking hypoglycemic symptoms and worsening glycemic control have prevented wide use of beta blockers in diabetics [1,3].
Worsening hyperglycemia is of particular concern, as numerous studies have shown that worsening insulin resistance increases CVD risk factors including inflammation, oxidative stress and endothelial function [4-8]. The recently published GEMINI trial-compared the selective beta-1 receptor antagonist, metoprolol, against the non-selective β- and α1-receptor antagonist, carvedilol, in patients with type 2 diabetes. Both drugs were effective in reducing blood pressure and were well tolerated [9]. Interestingly, carvedilol demonstrated a more favorable effect on factors associated with the metabolic syndrome compared to metoprolol. An investigator participating in the GEMINI trial completed an additional investigator initiated sub study to measure the effects of carvedilol and metoprolol on endothelial function. The investigators demonstrated that carvedilol significantly improved endothelial function compared to metoprolol [10]. To further test the hypothesis that carvedilol has unique beneficial effects on endothelial function, inflammation, and oxidative stress compared to metoprolol in patients with type 2 diabetes, we performed the following study.
2. METHODS
2.1. Patient Population
This study was conducted as a sub study to the GEMINI study [9]. Inclusion and exclusion criteria have been described in detail in the GEMINI publication. Briefly, twenty subjects with type 2 diabetes and uncontrolled hypertension despite current treatment with an Angiotensin Converting Enzyme Inhibitor (ACE) or an Angiotensin Receptor Blocker (ARB) were enrolled and randomized to receive either carvedilol or metoprolol. Verbal and written consent was obtained from each subject. The study was approved by Human Research and Review Committee (HRRC), the local Institutional Review Board at the University of New Mexico Health Sciences Center. All procedures were conducted in accordance with the HRRC and the Health Insurance Portability and Accountability Act policies. Clinical Trials Number: NCT00642434.
2.2. Study Design
The study design was identical to that of the parent study, and is described in detail elsewhere [9]. Subjects continued their ACE/ARB throughout the study. They were washed out of all other antihypertensive agents. Study medication was titrated to maximum dose. If blood pressure was not at the study protocol designated target of <130/80 mmHg, hydrochlorothiazide 25 mg was added. Amlodipine was added if blood pressure was still not at goal. Once goal blood pressure was reached, subjects were maintained on study treatment for five months.
2.3. Study Protocols and Procedures
All subjects underwent a baseline study, following a two week washout of all anti-hypertensives except for ACE/ARBs. All study procedures were repeated after five months of study intervention.
Subjects were admitted to the inpatient General Clinical Research Center in the evening. They received a standard ADA meal, and then began an observed 12-hour fast. The following morning, subjects underwent a blood test followed by a study of vascular endothelial function (see Section 2.5 Endothelial Function). Subjects then underwent a 75 gram oral glucose tolerance. Two hours later blood tests were repeated and endothelial function was retested.
2.4. Laboratory Analyses
Samples were taken to measure lipids, glycemic control (adiponectin, HbA1c). Glucose and insulin were used to calculate Homeostasis Model Assessment: Insulin resistance (HOMA-IR) [11]. Inflammation was assessed by highly sensitive C-reactive protein (hsCRP) and interleukin six (IL-6). Coagulation/thromobosis parameters were assessed by Plasminogen Activator Inhibitor one (PAI-1), fibrinogen, and homocysteine (HCY).
HCY was processed in pre-iced, 4-mL EDTA tubes, immediately placed on ice, and centrifuged. Concentrations were determined by Immulite Chemiluminescence, Diagnostic Products Corporation, (reference range, 5 - 15 mmol/L, GCRC labs, Albuquerque, NM). PAI-1 activity was measured using a Chromolize PAI-1 kit by biopool, Kit catalog number: 1106, (Method: A “Sandwich type” enzyme-linked immunosorbent assay), (reference range 2 - 50 IU/ml GCRC labs, Albuquerque, NM). HsCRP concentration was determined by immunometric assay, IMMULITE-high sensitivity CRP, (reference range: undetectable-1.1 mg/dl, GCRC labs, Albuquerque, NM). Glucose was processed in 8.5 mL SST, centrifuged and measured on the ACE instrument, (Hexokinase method) manufactured by Alfa Wassermann, Inc. West Caldwell, NJ, (reference range 79.3 - 129.2 mg/dL, GCRC core Lab Albuquerque, NM). Total cholesterol, LDL, HDL and triglycerides were measured by spectrophotometry, (reference range, <200 mg/dL, <100 mg/dL, >40 mg/dL, and <150 mg/dL, respectively, Tricore Reference Labs, Albuquerque, NM). HbA1c was measured by inhibition of latex agglutination, (DCA 2000 Hemoglobin A1c reagent kit, Bayer Corporation, USA), (reference range, 4.4% - 5.8%, GCRC labs, Albuquerque, NM).
Adiponectin was measured by ELISA, (reference range, 2 – 40 μg/mL, GCRC labs, Albuquerque, NM). High Sensitivity IL-6 was measured by ELISA, (reference range, condition dependent, calibration range, 0.156 - 10 pg/mL, GCRC labs, Albuquerque, NM). Insulin was measured by Immulite (Chemiluminescence), (reference range, 2 - 28.4 μIU/mL, GCRC labs, Albuquerque, NM).
2.5. Endothelial Function
Vascular endothelial function was assessed by measuring flow mediated dilation (FMD) of the brachial artery in response to hyperemia, using standard techniques [12]. Specifically, all studies were conducted in a temperature-controlled room. Subjects were placed comfortably in a supine resting position with the left arm immobilized for at least fifteen minutes prior to study procedures. A sphygmomanometer cuff was positioned above the antecubital fossa on the upper left arm. A baseline brachial artery diameter was measured using a 7.5 MHz linear array ultrasound. Diameter measurements were taken from one media-adventitia interface to the other. The brachial artery diameter was measured three times, and the average was recorded. Following baseline measurement, endothelial function was assessed following ischemic hyperemia. The cuff was inflated to 200 mmHg or 60 mmHg above systolic blood pressure for five minutes. The brachial artery diameter was remeasured at one minute post-deflation. After five minutes of recuperation, the subjects took 0.4 mg of sublingual nitroglycerin, and the brachial artery diameter measurements were repeated to evaluate endothelial independent dilation. Percent increase in brachial artery diameter from baseline following both postischemic hyperemia and nitroglycerine administration was calculated and represents the FMD endpoint for endothelial dependent and endothelial independent function respectively.
2.6. Statistical Analysis
Data were analyzed by SAS/STAT® Version 8.0 (SAS Institute Inc., Cary, NC). The data were analyzed using student’s t-tests and repeated measures ANOVA. Descriptive data were reported as a mean ± standard deviation. A p < 0.05 was considered significant.
3. RESULTS
Twenty subjects met enrollment criteria, completed all study visits, and were thus included in the final analysis. Nine subjects were randomized to metoprolol and eleven subjects were randomized to carvedilol. At baseline, there were no differences in parameters of glucose control, markers of inflammation, oxidative stress or endothelial function.
Labs
Following five months of treatment with study drugthere were no significant differences IL-6, hsCRP, or adiponectin (Table 1). Interestingly, PAI-1, an adipocytokine associated with thrombolysis, was significantly increased from baseline following five months of metoprolol treatment (19.5 ± 24 IU/mL versus 28.4 ± 26 IU/mL, respectively, p < 0.05). There were no significant changes in PAI-1 in the group treated with carvedilol (20 ± 12.5 IU/mL versus 24.5 ± 16 IU/mL, respectively, p = ns) (Table 1).
Additionally, while not reaching statistical significance, important trends suggest that that metoprolol and carvedilol may differ in their effects on insulin sensitivity and glycemic control. Specifically, subjects treated with metoprolol had a non-significant increase in HbA1c compared to baseline after five months of treatment (6.8 ± 0.8% versus 7.1 ± 1.1% respectively) and HOMA IR (94 ± 58 versus 130 ± 84 respectively) compared to those treated with carvediolol (HbA1c (6.6 ± 0.6% versus 6.5 ± 0.9% respectively), and HOMA-IR (86 ± 90 versus 93.7 ± 65 respectively) (Table 2).
Vascular data are shown in Figure 1. At baseline, there were no significant differences in resting brachial artery diameter or FMD between the metoprolol and carvedilol groups. Acute hyperglycemia led to an increase in resting brachial artery diameter in both groups at baseline. Following five months of treatment, there were no differences in resting brachial artery diameter compared to baseline in either group. However, the group receiving carvedilol demonstrated a non-significant trend toward increasing FMD compared to baseline. These changes were seen under both fasting and after acute hyperglycemia. Conversely, FMD in the fasting state was unchanged in the metoprolol group following five months of treatment. Interestingly, metoprolol treatment blunted acute hyperglycemia induced increases in FMD (p < 0.05).
Study drug was well tolerated in both groups, and neither group experienced any adverse effects throughout the course of the study.

Table 1. Changes in markers of inflammation following treatment with carvedilol versus metoprolol.
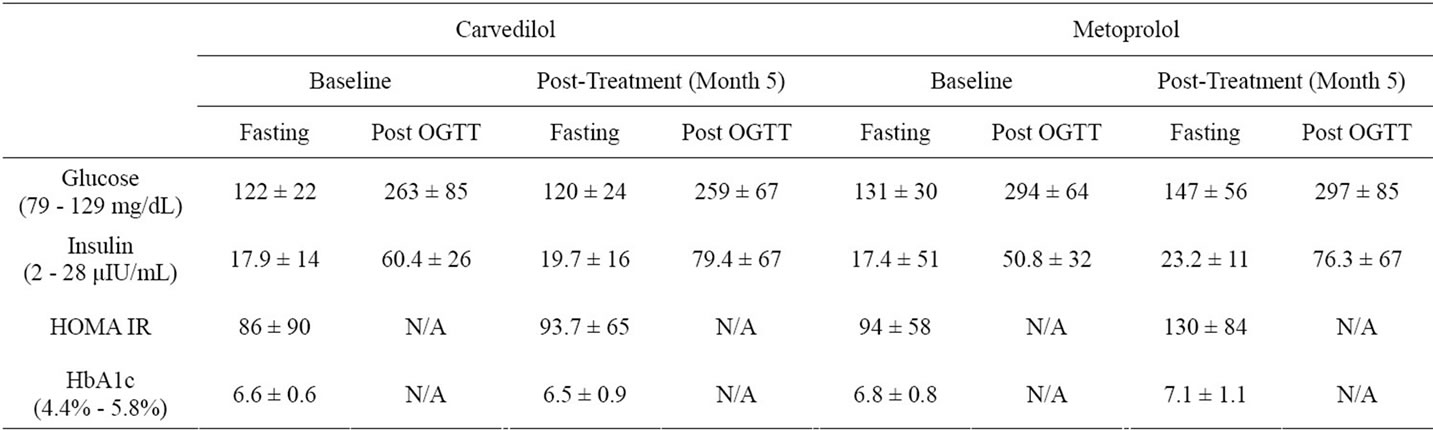
Table 2. Changes in markers of insulin resistance following treatment with carvedilol versus metoprolol.
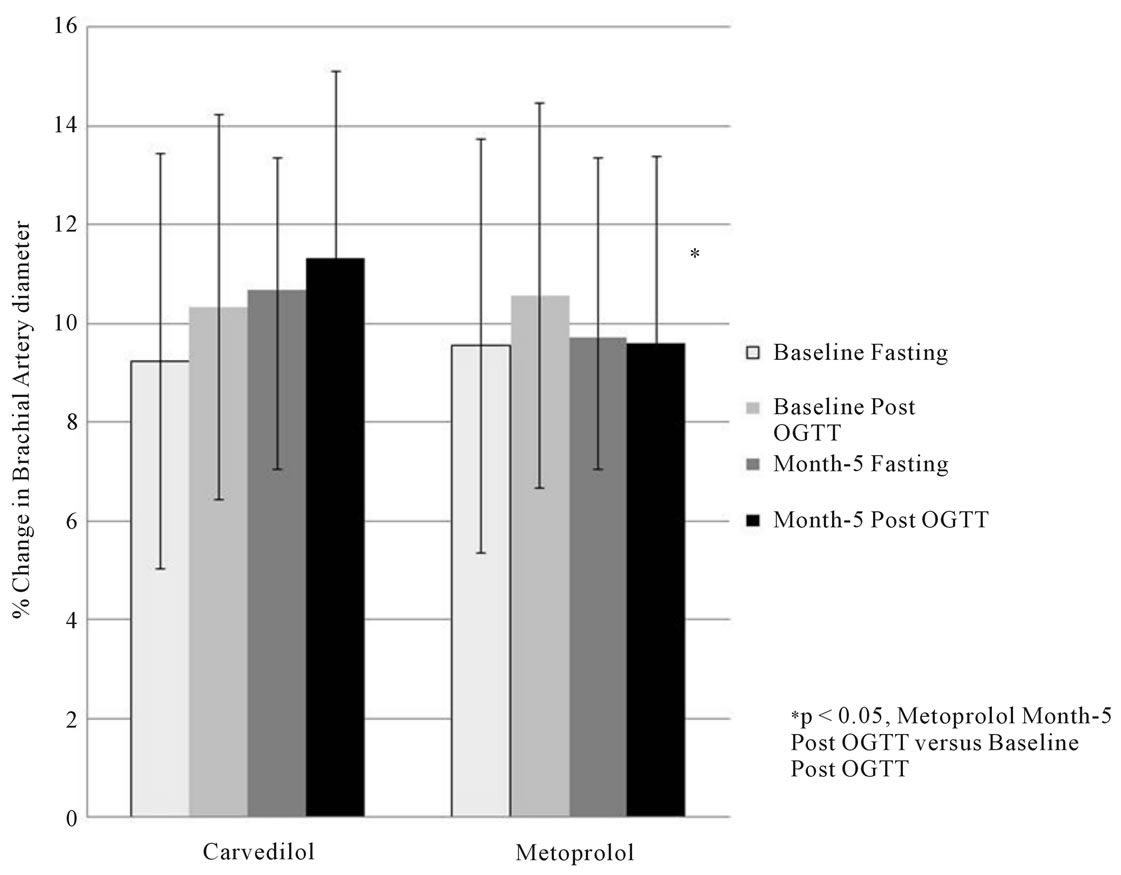
Figure 1. Change in brachial artery diameter following ischemic hyperemia before and after an OGTT at baseline and after 5 months of study drug.
4. DISCUSSIONS
In this study, we demonstrated that carvedilol and metoprolol are equally effective in reducing blood pressure and are well tolerated. However, carvedilol appears to have more beneficial metabolic and vascular effects compared to metoprolol. Specifically, subjects treated with carvedilol had lower PAI-1 activity compared to those treated with metoprolol. Additionally, two-hour post OGTT PAI-1 activity was lower in the group treated with carvediolol. PAI-1, is an antifibrinolytic protein. PAI-1 blocks the activation of plasminogen to plasmin thus preventing clot lysis. It is produced in numerous tissues including adipose tissues. Elevations in PAI-1 are associated with both CVD and insulin resistance [13, 14].
We did not find any significant differences in other novel CVD risk factors including inflammation and oxidative stress. However, numerous studies have demonstrated that carvedilol does indeed improve oxidative stress compared to other earlier or second generation beta blockers [15-18]. Our study may not have been powered to detect changes in inflammation and oxidative stress.
We did, however, find a trend toward worsening insulin resistance and glycemic control in subjects treated with metoprolol. Similarly, others studies, including GEMINI, have demonstrated that that carvedilol has beneficial effects on insulin sensitivity compared to other beta blockers [9,10].
4.1. Ultra Sound
Changes in FMD in the brachial artery did not differ by treatment group. However, there were trends suggesting that treatment with carvedilol may lead to improvement in FMD thus endothelial function compared to metoprolol. Previous studies have demonstrated similar results including another sub-study of the GEMINI Trial [19,20].
Interestingly, acute hyperglycemia two hours following a 75 gram glucose challenge led to increased FMD compared to fasting under baseline conditions. Reasons for this are not clear but could include the osmotic effect of hyperglycemia initially increasing extracellular blood volume. However, in our study, increases in FMD following acute hyperglycemia were blunted following five months of treatment with metoprolol, but were unaffected by treatment with carvedilol. Further investigation is warranted.
4.2. Limitations
There were several limitations to our study. The study groups were unequal. Twelve subjects were randomized to the carvedilol treatment arm and eight to the metoprolol arm. Computer randomization should have prevented group assignment inequality. Unfortunately, there were two distinct randomization methods used for our cohort. The initial seven subjects were randomized as part of the multicenter study, GEMINI [9]. Following enrollment closure of GEMINI, we continued to enroll fifteen subjects for this sub study. Our research pharmacist created a new randomized program. The two different systems were not matched leading to unequal randomization to study groups. Additionally, two subjects in the second part of the study did not complete all study visits and were not included in the final analysis.
5. CONCLUSION
We conclude that beta blockers are efficacious in reducing blood pressure and are well tolerated in patients with type 2 diabetes. However, metoprolol has more adverse metabolic effects compared to carvedilol including increasing PAI-1 activity and trends toward worsening insulin resistance and impaired endothelial function.
6. ACKNOWLEDGEMENTS
The authors would like to thank Lisa Toelle for providing editing, formatting and other technical assistance.


NOTES
Glaxo Smith Kline funded this investigator initiated project.