In Frail Elderly Patients, Low-Dose Gemcitabine over 6-Hour Infusion Is Equally Effective and Less Toxic Than the Standard Gemcitabine Protocol for Advanced Pancreatic Adenocarcinoma: A Randomized Phase II Trial ()
1. Introduction
Pancreatic cancer is one of the major worldwide health problems as it is the 11th most common cancer in the world [1]. It has the worst overall survival among all cancers ranking as the seventh most common cause of death from cancer worldwide [1]. Worldwide incidence and mortality of pancreatic cancer correlate with increasing age [1]. Most cases are diagnosed in the advanced stage as it has spread when a patient complains of tumor-specific symptom [2].
A landmark randomized phase III study conducted by Burris et al. comparing gemcitabine versus 5-fluorouracil in the treatment of advanced pancreatic cancer. They confirmed a statistically significant survival advantage favoring gemcitabine arm in terms of median and 1-year overall survival (5.7 months and 18% vs. 4.4 months and 2%, respectively; P = 0.0025) [3]. Gemcitabine became the standard regimen in patients with advanced or metastatic disease based on the previous study [3].
PRODIGE 4/ACCORD 11 Phase III trial stated the superiority of FOLFIRINOX regimen compared to gemcitabine as first-line therapy for metastatic pancreatic cancer as regard of overall survival (11.1 months vs 6.8 months for FOLFIRINOX and gemcitabine, respectively; HR = 0.57, P < 0.001) [4]. Due to the high toxicity of FOLFIRINOX, they exclude patients with performance status 2 or elderly patients (age more than 65 years) [4].
Another phase III clinical trial “MPACT trial” reported that the combination of gemcitabine and nab-paclitaxel was more effective than gemcitabine mono-therapy for metastatic pancreatic cancer [5]. A recent review article published by Macchini et al. (2019) [6] that mentioned the limitation of the MPACT study in respect of age, and they said: “This MPACT results reported overall acceptable toxicity even if, once more, data were not stratified by age”.
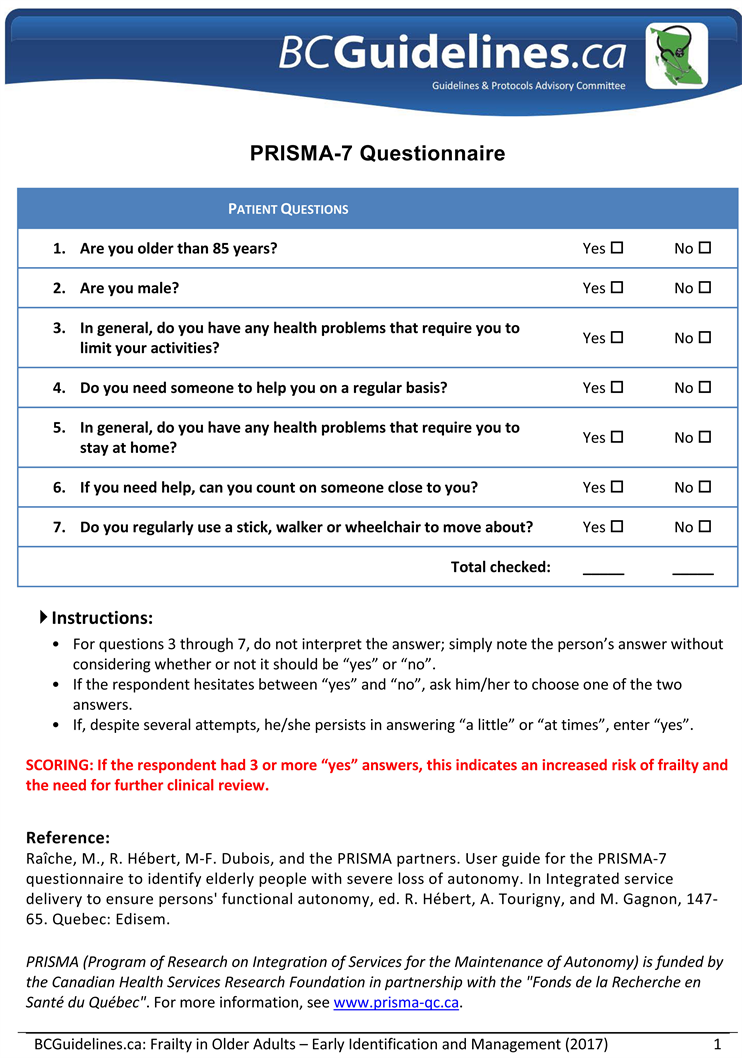
Treatment of the frail elderly patients is still challenging. “Frailty” is the accumulation of multiple physical and psychosocial deficits in the older person [7]. A gait speed < 0.8 m/s (taking > 5 seconds to walk 4 meters) or a timed-up-and-go-test (TUGT) > 10 seconds plus a score of ≥3 on the PRISMA 7 (Program of Research to Integrate the Services for the Maintenance of Autonomy 7) score indicates the frailty [8] [9]. Frail elderly people have diminished the capacity to compensate for stressors compared to people of the same chronological age, implying a state of elevated risk in the context of treatment decision-making [10]. Elderly patients are defined by the World Health Organization (WHO) as people older than 65 years. [11]
Gemcitabine has antitumor activity in elderly patients with locally advanced and metastatic pancreatic ductal adenocarcinoma in several studies [12] [13]. Also, in a review article, Higuera et al. recommended gemcitabine monotherapy for frail elderly patients [14].
Many Phase I studies were constructed trying to minimize the gemcitabine toxicity without decreasing its efficacy. These phase I studies [15] [16] [17] stated that the prolonged infusion of gemcitabine during 3-, 4-, 6-, and 24-hour at low dose levels in patients with advanced solid tumors were safe. Also, in these studies, the doses value between 180 and 450 mg/m2. The aim of our phase II trial was to compare the low dose gemcitabine over 6 hours to the standard gemcitabine protocol in terms of clinical benefit and survivals in the frail elderly patients with advanced pancreatic adenocarcinoma.
2. Methods
Sample Size
We started our trial without calculation of the sample size. However, we assessed all available patients (112 patients) for eligibility criteria during the study period (40 months), we assumed that is enough number for analysis based on that our sample size is much more than that of previous phase II randomized trial published by Sakamoto et al. who enrolled 25 patients and analysed 21 eligible patients.
Patient Selection
The eligibility criteria were the evidence of the advanced pancreatic cancer, both sexes, age of 65 years or older, frail patients (A gait speed < 0.8 m/s [taking > 5 s to walk 4 m] or a timed-up-and-go-test (TUGT test) > 10 seconds with a score of ≥3 on the PRISMA 7 score) [8] [9], an Eastern Cooperative Oncology Group (ECOG) performance status 2, patients not receiving prior chemotherapy, and adequate hematologic, hepatic, and renal functions. Relieving of obstructive jaundice either by stenting via endoscopic retrograde cholangiopancreatography (ERCP) or surgically by choledochojejunostomy or hepaticojejunostomy was allowed. Also, palliative radiotherapy for distant metastasis was allowed if indicated. Exclusions criteria were the evidence of second primary malignancy, concurrent local radiotherapy, and any psychiatric disease or social problem that would affect the compliance of participants. Also, patients with unknown tumor responses were excluded.
During the study period from May 2016 to September 2019, we assessed 112 patients for eligibility. Our manuscript reporting adheres to CONSORT guidelines for reporting clinical trials (CONSORT diagram: Appendix I, CONSORT checklist: Appendix II).
Our trial was approved by the ethics committee and institutional review board under the number of SECI-IRB-IORG0006563: No: 254 on 28 March 2016. The committee that approved the research confirmed that all research was performed in accordance with relevant guidelines/regulations. The informed consent was obtained from all participants and/or their legal guardians. The drugs were supplied by governmental and health insurance at the location of the study.
Study Design and Treatment Plan
Patients enrolled in this trial were randomly assigned by the corresponding author in a 1:1 fashion via closed envelope method to either receive gemcitabine of 1000 mg/m2 over 30-minute infusion on days 1, 8, and 15 of every 4-week cycle (standard protocol arm) or weekly low-dose (250 mg/m2) over 6-hour infusion (LD6H arm). The gemcitabine dose was reduced to 75% of the original cycle initiation dose, in the case of the following hematological toxicities: absolute granulocyte counts less than 500 × 106/L for more than 5 days, febrile neutropenia, platelets less than 25 × 109/L, or cycle delay of more than 1 week due to toxicity. Patients were categorized according to age (75 years is cutoff between the 2 age groups), gender (male vs. female), PRIMSA 7 score (score 3/4 vs. score 5 - 7), tumor grade (1/2 vs. 3), tumor location (pancreatic head, body, tail, or diffuse), liver metastasis (yes vs. no), lung metastasis (yes vs. no), and the number of metastatic organ(s) (0/1 vs. two or more). The crossover between the two arms was not allowed. Patients were continued to receive either regimen until disease progression, unacceptable toxicity, study end, or on patient demand.
Assessments
Baseline evaluation included a detailed history, physical examination, hematological counts, renal, and hepatic functions tests. Assessment of performance status was done based on the ECOG scale. Assessment of frailty via gait speed, TGUT test and PRIMSA 7 score (Appendix III). Radiographic tumor assessments by computed tomography or magnetic resonance imaging were performed at baseline, every 8 weeks, and in the presence of any suspicion of tumor progression. The response was assessed by the investigators according to the Response Evaluation Criteria in Solid Tumors (RECIST 1.1) [18]. At each gemcitabine administration, all adverse events were assessed according to the Common Terminology Criteria for Adverse Events (CTCAE 4.03) [19].
Statistical Analysis
The primary end-points were progression-free survival (PFS), defined as the time from the start of treatment to disease progression or death from any cause, whichever came first, and safety through assessment toxicity profile based on CTCAE 4.03. Secondary end-points were overall survival (OS; defined as the time from the start of treatment to date of death from any cause, or date of last follow up, whichever came first.), overall response rate (ORR; defined as sum of rates of complete response (CR) and partial response (PR) to chemotherapy), and disease control rate (DCR; defined as sum of rates of stable disease (SD), complete response, and partial response). Univariate analysis was used through the presentation of continuous variables as median and range. Categorical variables are presented as frequency and percentage. Bivariate analysis was done to compare categorical variables using Chi-Square test or Fisher Exact test when appropriate. Kaplan-Meier method was used to estimate the survival time distribution and the median survival of each treatment group. The treatment difference between the two groups was assessed by a log-rank test. Hazard Ratios (HRs) and 95% confidence intervals (CIs) were determined by using a cox proportional hazards model. Factors were re-assessed by multivariate analysis by using Cox regression analysis. Median follow up time for all patients was derived from the reverse Kaplan-Meier method. A P-value less than 0.05 is considered as a cut off of significance. SPSS version 21 (SPSS Inc. Chicago, IL, USA) was used in the storage and analysis of data [20].
3. Results
Patients’ Chart
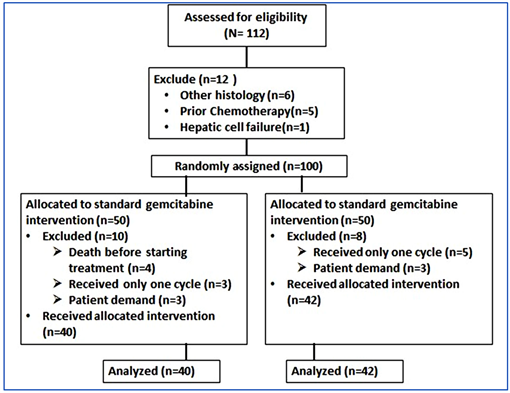
During the study period from May 2016 to September 2019, we assessed 112 patients for eligibility. Ten patients were excluded from standard arm due to death before starting treatment (n = 4), lost follow up after 1st cycle without assessment (n = 3), and patient demand (n = 3); while eight patients from the experimental arm were excluded from analysis, five of them died before first assessment and the remaining three patients withdrew their consent. The last day of the follow up was 30 September 2019 as the study was completed and prepared for analysis. Eighty-two patients were analyzed for study endpoints; 40 patients in standard arm and 42 patients in LD6H arm. CONSORT diagram shows the patients’ chart (Appendix I).
Demographics and Patients’ Characteristics
The data is shown in Table 1. The median ages were 70.5 and 69.5 years for the standard arm and LD6H arm respectively, with a range of 65 - 81 years for each. The gender was fairly distributed within the two arms (55% males in standard arm and 52.4% in LD6H arm). The metastases affected only one organ in 19 cases (47.5%) in the standard arm vs. 24 cases (57.1%) in the LD6H arm. The liver was the most common site of distant metastasis (75% for standard arm and 69% for LD6H arm).
Efficacy
Response Rates
The partial response rate was 17.5% in the standard arm and 21.4% in the LD6H arm. One-quarter (25%) of patients receiving the standard protocol remained stable versus 21.4% in the LD6H arm. There was no case of complete response (CR) in both arms. There was no significant difference between the standard group and LD6H group as regard ORR and DCR (17.5% vs. 21.4% respectively; p = 0.654 for ORR and 45% vs. 42.9% respectively; p = 845) (Table 2).
Survival End-Points
Progression-Free Survival (PFS)
After a median follow-up of 9 months, the median PFS was 5 months in both groups, with 95% CI, 2.78 to 7.22 for standard group and 95% CI, 3.89 to 6.12
![]()
Table 1. Demographics and baseline characteristics of enrolled patients (n = 82) received standard gemcitabine protocol or gemcitabine of low dose over 6-hour infusion.
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; LD6H, low dose over 6 hours; PRIMSA 7, Program of Research to Integrate the Services for the Maintenance of Autonomy. *The grade was not known because the tissue diagnosis couldn’t be done.
![]()
Table 2. Response rates according to recist criteria in patients received standard gemcitabine protocol or gemcitabine of low dose over 6-hour infusion.
Abbreviations: CR, complete response; DCR, disease control rate; LD6H, low dose over 6 hours; ORR, overall response rate; PR, partial response; RECIST, response evaluation criteria in solid tumors; SD, stable disease.
for LD6H group; log-rank p = 0.908; unadjusted HR 1.07; 95% CI 0.60 to 1.90 (Figure 1(a)). Adjusted HR through multivariate analysis confirmed the lack of significant effect of chemotherapy type on PFS (adjusted HR 1.08; 95% CI 0.65 to 1.7; p = 0.762) (attached complementary file 1). Subgroup analysis within the study arms revealed the absence of any significant effect of any subgroup of the examined parameter on PFS (Figure 2(a)).
Overall Survival (OS)
Median OS was not significantly different between the two arms: 10 months; 95% CI 8.95 to 11.05 in the standard protocol arm versus 8 months; 95% CI 6.41 to 11.59 in LD6H arm; unadjusted HR 1.25, 95% CI 76 to 2.05; log-rank p = 0.331; Figure 1(b). Regarding OS, there was an absent of any significant interaction between the studied factors including chemotherapy type. The adjusted HR for chemotherapy type was 1.45; 95% CI .85 - 2.46; p = 0.170 (complementary file 2). Also, no subgroup of the examined parameters had a significant effect on OS as shown in the forest plot Figure, titled Figure 2(b).
Adverse Events
Totally, patients in standard protocol arm received a number of 205 cycles of treatment (mode, 6; range 2 - 12) versus 226 cycles (mode 3, range 2 - 17) in the LD6H group (Table 1). Most hematologic and nonhematologic adverse events were grade 1 or 2 intensity (Table 3). For all grades, fatigue was the most common nonhematologic adverse effect in both groups (37.5% in standard protocol group and 31% in LD6H group) followed by anorexia (30%) in standard protocol group and hypotension (17.5%) in LD6H group. The thrombocytopenia was the most frequently reported hematologic side effect in standard protocol and LD6H arms (45% and 38.1% respectively) (Table 3).
![]()
Figure 1. Kaplan-Meier plot for (a) PFS and (b) OS. CI: confidence interval; HR: hazard ratio; OS: overall survival; PFS: progression-free survival.
![]()
Table 3. Toxicities according to CTCAE (version 4.03) in patients received standard gemcitabine protocol or gemcitabine of low dose over 6-hour infusion.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate transaminase; CI, confidence interval; CTCAE, the common terminology criteria for adverse events; LD6H, low dose over 6 hours; OR, odds ratio. *p value was calculated by the Ficher’s exact test as all items having cell with expected count less than 5.
Chi-square test was used as the comparative test between two arms regarding grade 3/4 toxicity (Table 3). LD6H regimen had a significantly lower incidence of grade 3/4 fatigue and hypotension when compared to the standard arm (4.8%
![]()
Figure 2. Prespecified subgroup analysis by forest plot, with HRs for (a) PFS and (b) OS. CI: confidence interval, HR: Hazard Ratio; OS: overall survival; PFS: progression free survival; PRIMSA: Program of Research to Integrate the Services for the Maintenance of Autonomy.
vs. 22.5% respectively, p = 0.024 for fatigue, and 2.4% vs. 27.5% respectively, p = 0.027 for hypotension). For hematologic adverse events, there was a significantly lower incidence of grade 3/4 thrombocytopenia in LD6H arm when compared to that in the standard protocol arm (2.4% vs. 20%, p = 0.012). Grade 3/4 neutropenia was much lower in LD6H protocol than in the standard protocol (2.4% vs. 22.5% respectively, p = 0.006). There were no treatment-related deaths. A 75% dose reduction was required in 4 patients in standard protocol arm, while no dose reduction was required for gemcitabine in patients received the low-dose regimen.
Our raw data is available in the attached commentary file 3.
4. Discussion
The treatment of metastatic pancreatic adenocarcinoma is slowly progressive in spite of its worst survival among all cancer [1]. The gemcitabine 1000 mg/m2 over 30-minute infusion on days 1, 8, and 15 of 4-week cycle became the standard regimen in patients with advanced or metastatic pancreatic cancer based on the randomized phase III study conducted by Burris et al. who proved a statistically significant survival advantage of gemcitabine arm when compared to 5-fluorouracil in term of median and 1-year OS [3]. Two large randomized studies documented the superiority of FOLFIRINOX regimen in PRODIGE 4/ACCORD 11 Phase III trial [4] and gemcitabine and nab-paclitaxel combination in MPACT phase III clinical trial [5] over gemcitabine alone. However, both trials did not take the factor of frailty into consideration. Also, in both trials, the age was not the parameter of patients’ stratification (elderly patients and PS grade 2 were excluded from in PRODIGE 4/ACCORD 11 trial and insufficient data about outcome in relation to the age in MPACT trial).
To the best of our knowledge, our study is the first randomized phase II study comparing the standard gemcitabine protocol with low-dose gemcitabine over 6-hour infusion in frail elderly patients with advanced pancreatic cancer. In our trial, there was no significant difference between the standard group and LD6H group as regard ORR and DCR (17.5% vs. 21.4% respectively; p = 0.654 for ORR and 45% vs. 42.9% respectively; p = 845). Also, there is no significant difference in median PFS, 5 months in both groups, p = 0.908; unadjusted HR 1.07; 95% CI 0.60 - 1.90; adjusted HR 1.08; 95% CI 0.65 - 1.7; hazard p = 0.762). Also, the result of OS is insignificant (median OS:10 months; 95% CI 8.95 - 11.05 in standard arm versus 8 months, 95% CI 6.41 - 11.59 in LD6H group; unadjusted HR 1.25; 95% CI 76 to 2.05; p = 0.331). Subgroup analysis within the study arms revealed the absence of any significant effect of any subgroup of the examined parameters on PFS and OS. The low-dose regimen has a significantly lower incidence of adverse effects grades 3 or 4 when compared to the standard regimen: (4.8% vs. 22.5%; p = 0.024 for fatigue, 2.4% vs. 27.5%; p = 0.027 for hypotension, 2.4% vs. 20%; p = 0.012 for each anemia and thrombocytopenia, 22.5% vs. 2.4%, p = 0.006 for neutropenia).
Our results agree with a phase II study conducted by Sakamoto et al. [21] who documented that low dose gemcitabine is less toxic and equally effective to the standard gemcitabine. However, this comparison is not dependable because of the presence of meaningful differences in patients and methods between the two studies as we used prolonged infusion time (6-hour) with frailty as eligible criterion and, the age and PRIMSA 7 score parameters as indicators of comparison, while they did not. The incidence of grade 3/4 adverse events in the low-dose arm of our study is higher than that of the low-dose arm of Sakamoto study [21]. It may be due to the biological nature of our patients as the frail people have diminished the capacity to compensate for stressors compared to people of the same chronological age [10].
Matsumoto et al. [22] proved that the low-dose gemcitabine is superior to the best supportive care (BSC) in elderly (they do not report frailty.) In spite of using a low dose gemcitabine in elderly, we cannot compare our results with Matsumoto et al. [22], because the comparator arm is different (standard gemcitabine protocol in our study and BSC in Matsumoto study). Also, the dosage of the experimental arm is different in spite of named low-dose gemcitabine (250 mg/m2 in our study and 600 - 800 mg/m2 in Matsumoto study).
The small sample size is one of the limitations of our study. Also, our sample size depends on availability of the eligible cases, not on the prior statistical calculation of that size.
5. Conclusion
Low-dose gemcitabine over 6-hour infusion is equally effective and less toxic when compared to standard gemcitabine protocol for frail elderly patients with advanced pancreatic adenocarcinoma. So, we recommend low-dose gemcitabine for frail elderly patients with advanced pancreatic cancer.
List of Abbreviations
BSC: Best Supportive care.
CIs: Confidence Intervals.
CR: Complete Response.
CTCAE: Common Terminology Criteria for Adverse Events.
DCR: Disease Control Rate.
ECOG: Eastern Cooperative Oncology Group.
ERCP: Endoscopic Retrograde Cholangiopancreatography.
FDR: Fixed-Dose Rate.
HR: Hazard Ratio.
LD6H*: Low-Dose over 6-Hour.
OS: Overall Survival.
PFS: Progression Free Survival.
PRIMSA 7: Program of Research to Integrate the Services for the Maintenance of Autonomy.
PR: Partial Response.
PS: Performance Status.
RECIST: Response Evaluation Criteria in Solid Tumors.
SD: Stable Disease.
SPSS: Statistical Package for Social Sciences.
TUGT: Timed-Up-and-Go-Test.
Vs.: Versus.
WHO: World Health Organization.
*LD6H is not standard abbreviation, but it is abbreviated as it appears more than three times in the text
Appendix I: Consort Diagram
Appendix II: Consort 2010 Checklist
 Consort 2010 checklist of information to include when reporting a randomised trial*.
Consort 2010 checklist of information to include when reporting a randomised trial*.
*We strongly recommend reading this statement in conjunction with the CONSORT 2010 Explanation and Elaboration for important clarifications on all the items. If relevant, we also recommend reading CONSORT extensions for cluster randomised trials, non-inferiority and equivalence trials, non-pharmacological treatments, herbal interventions, and pragmatic trials. Additional extensions are forthcoming: for those and for up to date references relevant to this checklist, see http://www.consort-statement.org/.
Appendix III: Frailty-Prisma 7
Complementary File 1: Multivariate Analysis for PFS
Complementary File 2: Multivariate Analysis for OS