1. Introduction
Polymers like polybutadiene, polyisoprene and SBR present some disadvantages on their application due to their high permeability to gases and their low resistance to oil exposure. In order to circumvent these disadvantages, chemical modifications on their polymeric structure have been proposed, such as the introduction of different functional groups [1] [2]. The introduction of epoxy groups on the polymeric chain is one of the best ways to modify polydienes, generating materials with reduced unsaturation levels, with properties like lower air permeability, higher oil, solvents and shearing resistances and better compatibility with other polymers such as PVC. This allows their application on the manufacture of tires, adhesives with high melting point and surface coatings [3].
Varying the epoxidation degree, it is possible to obtain rubbers with different properties. The epoxidation degree must lie between 25% and 50%, as higher epoxidation degrees tend to elevate polymers glass transition temperature (Tg) and make them insoluble, due to the increase in chain polarity [4].
The epoxidation of unsaturated polymers is a very well-known reaction and peracids are commonly employed as oxygen sources [5]. Other usual oxygen donors are hydrogen peroxide [6] and tert-butylhydroperoxyde (TBHP) [7] [8] [9], which can react in the presence of metallic catalysts [10]. Besides costs and manageability, the two major factors that influence the choice of the oxidant are the nature of the by-product formed and its oxygen content. Obviously, environmental issues are important, which results in the necessity of reusing the formed by-products. In case of TBHP, the only by-product is terc-butanol, which can be recycled by reacting with hydrogen peroxide [11] [12]. Classic methods using peracids present problems related to the quantity of oxidant employed and handling complexities, such as the amount of residues inherently produced and their treatment, and corrosion issues due to reagent acidity.
The principal role of metallic catalysts is to withdraw electrons from the oxidant, yielding a species that is more likely to be attacked by nucleophiles as olefins. Due to this role as Lewis acid, normally metals with high oxidation states are used. This Lewis character is also influenced by ligands around the metal. However, the role of ligands can only be observed in initial states of the reaction due to the rapid destruction of them in the oxidative medium. Catalysts with ligands strongly bonded to the metal center normally present low activity, probably due to unfavorable formation of a complex between the metallic precursor and the oxidant [13].
Among the most used metals in the epoxidation of olefins, that can be either functionalized or not, are Ti, Zr, V, Mo and W [7] [14] - [23]. Molybdenum has a special catalytic importance, since its complexes are found in higher oxidation states, which facilitates the formation of catalytic species [16] [17] [18] [19] [24]. The chemistry of hexavalent molybdenum is dominated by oxo ligands. The high oxidation state of molybdenum requires the presence of electron-donor ligands in order to stabilize the complexes by increasing the electron density on the metal. In some cases, TBHP is a better oxidant than H2O2 due to its superior solubility in non-polar solvents [7] [8] [15] [16] [19] [23], besides its higher reactivity with molybdenum complexes. One of the most employed molybdenum precursors in the epoxidation of olefins is bis(acetylacetonate) dioxomolybdenum (VI) [MoO2(acac)2] [19], which has a better activity when TBHP is used as oxygen source.
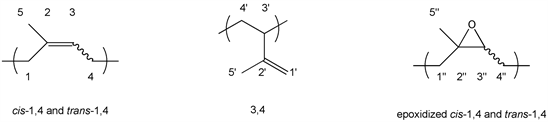
Polyisoprene is obtained from the polymerization of isoprene, which leads to several isomeric structures, shown in Figure 1. The relative proportion of each isomer can be determined by 1H-NMR [25].
The isomers have different applications. Cis-1,4-polyisoprene is used in the fabrication of tires and other rubbery materials. Due to its structural similarity with natural rubber, synthetic cis-polyisoprene and natural rubber have similar
![]()
Figure 1. Isoprene monomer and the different forms of polymerized isoprene.
applications [26]. Trans-1,4-polyisoprene is a crystalline thermoplastic resistant to abrasion and attrition, and due to its excellent mechanical properties, it can be used in orthopedic appliances, insulation, coating and as golf balls cover [27].
In this work, we have quantified the effect of the catalytic system [bis(acetylacetonato)dioxomolybdenum(VI)]-tert-butylhydroperoxide [MoO2(acac)2-TBHP] in polyisoprene epoxidation using a two level fractional factorial experimental design which minimizes experimental efforts while maximizing the amount of information obtained from the experimental system [28]. The experimental variables were catalyst and TBHP concentration, reaction temperature and time. The experimental design allows us to precisely quantify the influence of the experimental variables over the product final properties, in order to select conditions in which can be achieved higher selectivities with minor production costs.
2. Experimental
2.1. Equipments
2.2. Reagents
TBHP 70% (Merck) was extracted from the aqueous solution with toluene and its concentration was measured as described below. Commercial polyisoprene (Mn = 441,000 D, Mw = 704,000 D) was purified as described below prior to epoxidation reactions. All other chemicals were commercial grade and used as received.
2.3. Catalyst Preparation
The catalytic precursor MoO2(acac)2 was synthetized according to the classical method [29] : ten grams of the corresponding oxide were refluxed with 50 mL of 2,4-pentanodione for 18 h. The mixture was filtered, and the solution was added to 150 mL of petroleum ether, which was chilled for 1 h in an ice bath. An orange-yellow powder was formed, which was filtered and washed several times with petroleum ether. For catalytic reactions, the appropriate amount of catalyst was dissolved in toluene, under argon.
2.4. Purification of Polyisoprene
Commercial polyisoprene, 20 g, was dissolved in 200 mL of THF, under stirring. The solution was filtrated to eliminate some unsolved microgels that could be present. The solution was then added dropwise to 2 L of 95% ethanol, inducing the precipitation of the polymer. The polymer was pressed and dried in a vacuum oven until constant weight. It was then characterized by 1H and 13C NMR and IR. The spectra were in accordance with the literature [16] [17] [18]. 1H NMR showed that the polyisoprene had the following composition: 10% 3.4; 20% 1.4 trans and 70% 1,4 cis.
2.5. Extraction of the Oxidant
TBHP was supplied in aqueous solution. In order to use it in toluene solutions, it must be previously extracted. In an extraction funnel, 50 mL of commercial aqueous solution of TBHP 70% and 85 mL of toluene were vigorously shaken. The bottom aqueous phase was discarded, and the upper organic phase stored in a closed flask. Its concentration was measured by 1H NMR after the determination of the density of the solution, by the following method. The TBHP/toluene (mole/mole) ratio, R, can be obtained by 1H NMR by normalizing the area of their methyl peaks, Equation (1), where A is the area of the peaks and n the number of moles.
(1)
The density of the solution, its mass divided by its volume, can be calculated in terms of molar masses, Equation (2), where ρ is the density of the solution,
the molar mass and V the solution volume.
(2)
Replacing Equation (1) into Equation (2), the solution density can be obtained as function of the molar ratio, Equation (3).
(3)
Therefore, the number of mols of TBHP in one mL of solution, can be calculated by Equation (4).
. (4)
2.6. Catalytic Reactions
Blank tests, either in the absence of catalyst or TBHP were performed, and samples showed no modification on both 1H NMR and IR spectra. The catalytic reactions were performed starting from a stock solution of polyisoprene 5% (w/V) in toluene, using samples of 20 mL, under argon. Since after the polymerization reaction one double bond from each isoprene monomer remains, the starting number of double bonds was always 1 g/68 g∙mol−1 (=0.0147 mol). To polymer solutions, the necessary amount of catalyst in toluene was added. In an ice bath, TPHP was slowly added and the system was heated in a thermostatic bath, previously regulated to the reaction temperature. The reactions were conducted for predetermined times under argon and magnetic stirring and then quenched with Na2SO3. The products were then precipitated in ethanol containing 2,6-di-tert-butyl-4-methylphenol (BHT). After pressing and drying in a vacuum oven until constant weight, the samples were characterized by 1H and 13C NMR and IR.
2.7. 1H NMR Determination of Epoxidation Degree
The amount of epoxide was calculated using 1H NMR, from the ratio of the areas (A) of the hydrogens from oxirane rings (at 2.7 ppm) and the starting area of double bonds (at 4.7 and 5.2 ppm), as already described [30], using Equation (5).
. (5)
2.8. Experimental Design
To investigate the influence of the reaction conditions on the degree of epoxidation, it was used fractional factorial experimental design with two levels for each experimental variable. The experimental variables were catalyst ([Mo]) and oxidizing agent ([TBHP]) concentrations, calculated over molar concentration of polymer double bonds, reaction temperature and time. Since there were four experimental variables to investigate, a full two levels factorial experimental design would require a total of sixteen experiments. To minimize experimental cost, we use a half factorial design of eight experiments.
The experimental conditions have been chosen from a previous work in which VO(acac)2 was employed as the catalyst [9] The best reaction conditions with that catalyst were obtained using 150% of TBHP, 1% of VO(acac)2 and toluene as solvent, under reflux and magnetic stirring for one hour. However, using these conditions with MoO2(acac)2, high degree of epoxidation was obtained, and undesirable polymer reticulation occurred, making it unfeasible to quantify and characterize the products. Other preliminary tests were done in milder conditions, which proved to be more suitable for this catalytic system. The higher and lower values of the experimental variables are shown in Table 1.
![]()
Table 1. Reaction variables maximum and minimum values.
*Molar proportion to double bonds present in polymer.
The experimental variables, zi, were normalized within the [−1, +1] interval according to Equation (6), where zi denotes the actual variable values, the subscripts max and min stand for the maximum and minimum values used in the experimental design and xi is the normalized variable value. This normalization is useful when comparing variables with different sizes, such as catalyst concentration, that varies from 0.1 to 0.5 mol%, and temperature, that varies from 60˚C to 80˚C, all variables would be in the [−1, +1] range [28]. The experimental matrix of the half factorial experimental design is presented in Table 2.
(6)
The product degree of epoxidations were related to the reaction conditions using Equation (7), where y is this physical-chemical property, xi and xj are normalized reaction conditions, n is the number of independent variables, a0 is the independent parameter, and ai and aij are model parameters related to the linear and the interaction of two variables effects.
(7)
The estimation of parameters from Equation (7) was performed using least squares regression, using the software Statistica 8.0 (StatSoft). Statistical Student’s t-test was performed to evaluate parameter significance. The parameter was removed from the Equation (7) model when its confidence was lower than 95%.
3. Results and Discussion
3.1. 1H NMR Characterization
According to the literature [25], the peaks presented in Table 3 are relevant for calculations involving the epoxidation of polyisoprene.
Since selectivity in epoxidation is favored by the electronic environment of the double bonds [31], 1,4 double bonds are preferentially epoxidized, maintaining the area of the 3,4 units (1’ and 2’) constant. After epoxidation, a new sign is also observed at 1.3 ppm due to methyl groups near oxyrane rings (5’’). As epoxidation degrees increase, the area of peaks attributed to 1,4 double bonds (3) decreases, while the area of a new peak at 2.7 ppm (3’’) increases proportionally. Small peaks that could be attributed to ring opening or chain breaking, such as alcohols, ethers or acids were observed in some cases.
![]()
Table 2. Matrix of experimental conditions (actual and normalized values).
*Molar proportion to double bonds present in polymer.
![]()
Table 3. Relevant peaks for the calculation of epoxidation degree.
3.2. 13C NMR Characterization
Epoxide rings can also be observed by 13C NMR. According to the literature [32], the new peaks are attributed as shown in Table 4.
The selectivity of the reaction can again be stated as no peaks that could be attributed to carbonylic or hydroxyl-bonded carbons were seen.
3.3. Infrared Characterization
The bands that could be attributed are: 830 cm−1 (cis-1,4 C=C-H deformation), 1648 cm−1 (C=C stretching), 1384 cm−1 (CH3 deformation), 1455 cm−1 (CH2 deformation) and 881 cm−1 (oxyrane ring). This last one increases as epoxidation degrees increase, as well as the band at 830 cm−1 decreases. Very weak bands due to the formation of carbonylic and hydroxyl-bonded carbons could be seen for some reactions.
3.4. Quantitative Analysis of the Epoxidation Reaction
The results obtained in the epoxidation of polyisoprene are presented in Table 5,

![]()
Table 4. 13C NMR peaks of epoxidized polyisoprene.
![]()
Table 5. Results of catalytic tests with MoO2(acac)2 and TBHP.
aDetermined by 1H NMR, Equation (5).
where the degree of epoxidation was calculated from 1H NMR analysis according to Equation (5).
The degree of epoxidation was related to the reaction conditions according to Equation (7), whose parameters are presented in Table 6. Since it was done a half factorial experimental design, only two independent interaction effects between two variables (aij) would be quantifiable. Furthermore, these two effects could be confused with interaction effect between other two variables. However, with the experimental data available none of the interaction effects were statistically significant, leaving a better model with solely the linear effects (ai). All linear effects were statistically significant, indicating that all reaction conditions variables influence the degree of epoxidation.
It can be observed that the empirical model of Equation (7), Table 6 gives a good adjustment of the epoxidation degree as function of reaction conditions. As shown in Figure 2, the experimental data as function of the calculated values are very close to and well distributed around the line y = x.
The parameter a0 (Equation (7), Table 6) gives the average value of the
![]()
Figure 2. Experimental and calculated (Equation (7)) epoxidation degree.
![]()
Table 6. Equation (7) parameters relating epoxidation degree and reaction conditions.
epoxidation degree, while the a1, a2, a3 and a4 are the linear effects of the temperature, oxidant (TBHP) concentration, catalyst (Mo) concentration and reaction time, respectively. These parameters are all significant, meaning that all variables influence the epoxidation degree. The positive values of the parameters indicate that and when one of these variables increases the degree of epoxidation will increase. The parameter value quantifies the growth of the epoxidation degree value with the increment of the dimensionless reaction variable. The parameters a2 and a3 present the same value, indicating that the oxidant and the catalyst concentration have the same influence over the epoxidation degree. However, it is noteworthy that the parameters were obtained from the normalized reaction variables; therefore, in the regular scale, increasing catalyst concentration in 0.2 mol% gives the same effect as increasing the oxidant concentration in 10 mol%. The effect of the reaction variables over the degree of epoxidation can be better observed in Figure 3. More specifically, the results indicate that, when the reaction temperature increases 10˚C, the epoxidation degree will raise 9.25%, while the degree of epoxidation will increase 6.25% if either the oxidant concentration varies 10 mol% or the catalyst concentration varies 0.2 mol%. For the reaction time, an increase in 2 h will generate an increment of 10.75% in the epoxidation degree.
![]()
Figure 3. Epoxidation degree as function of the catalyst concentration varying temperature and oxidant concentration at (a) 1 hour reaction and (b) 5 hours reaction.
4. Conclusions
The use of the catalytic system MoO2(acac)2-TBHP for the epoxidation of polyisoprene was studied. The system was active and selective under the conditions employed. By Infrared analysis, it was possible to observe a very small amount of hydroxy-bonded and carbonylic carbons, which were not detected by 1H and 13C NMR, and so were assumed as being minor products.
Through a half factorial experimental design, it was possible to identify the influence of the reaction variables, temperature, oxidant (TBHP) concentration, catalyst (Mo) concentration and reaction time, on the epoxidation degree. The variables linear effects were all positive, indicating that if they are increased the epoxidation degree will also be increased. Therefore, if it is necessary to obtain an elevated epoxidation degree of polyisoprene, it would be better to use the higher level of the reaction variables, taking into account the experimental feasibility, due to low solubility of the epoxidized polymer when it reaches a degree of epoxidation close to 50%.
Acknowledgements
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001. The authors also thank PRONEX/CNPq/FAPERGS 10/0050-6 and CNPq 310967/2009-0) as well as FAPERGS, PROPESQ/UFRGS and FUGAPESQ (2019/230864) for financial support and fellowships.
NOTES
1H and 13C NMR analyses were performed at ambient temperature on a Varian VNMRS 300 MHz spectrometer, in CDCl3 and using TMS as internal reference. IR spectra were obtained in a Bruker Alpha-P FTIR/ATR equipment with 32 scans. Samples were dissolved in CHCl3 and a film was obtained by evaporation of the solvent on a KBr cell.