New Ionic Liquids with Buffering and Chelating Abilities for Enzyme Engineering ()
1. Introduction
Enzymes catalyze a wide variety of reactions best in heavy-metal-free aqueous environments and at physiological pH with exquisite selectivity and stereospecificity [1] . Thus, an appropriate buffer is needed to closely regulate the ionization state of ionizable groups of the enzyme in both aqueous and non-aqueous media. We have synthesized a new class of ionic liquids (ILs) with buffering behaviour that are referred to as IL buffers, which can be used for control of ionization state of enzymes in non-aqueous media [2] . Remarkable buffer dependence of the catalytic activities has been observed in hydrogenation of olefins [2] and selective hydrogenation of unsaturated aldehyde [3] in organic solvents and in ILs. Organic solvents are widely used with enzymes to improve the solubility of hydrophobic reactants and/or products and to shift reaction equilibria from hydrolysis toward synthesis [4] - [9] . With the emergence of ILs, the use of ILs as a new type of non-aqueous medium may offer a convenient solution to both the solvent emission and the catalyst recycling problem [5] [9] - [22] .
Increasing water contamination by heavy metals has prompted investigations to find ways to clean the environment and also to understand the mechanisms leading to metal toxicity, among which is enzyme inhibition. This inhibition was most often attributed to the reaction of the metal ions with the thiol groups of cysteine residues of the enzyme, resulting in the formation of mercaptides [23] [24] .
It was reported that peroxidases are inhibited by heavy metal ions at higher concentrations [25] and Hg2+ ion is listed as the most effective inhibitor [26] [27] . Konyaeva et al. reported that Hg2+ chelators like EDTA can protect hemoglobin against Hg2+-induced denaturation and precipitation [28] .
Like most biological buffers in use today, IL buffers were developed only for keeping the pH of a solution constant, which cannot protect enzymes against metal-induced denaturation and precipitation. We then designed and synthesized new ILs with both buffering and chelating abilities for enzymatic reactions in heavy metal containing aqueous system and in IL system.
2. Materials and Methods
2.1. Materials
Candida antarctica lipase B (CALB, 10.9 U∙mg-1), horseradish peroxidase (HRP, 150 U∙mg-1), bis-tris-propane (BTP), guaiacol and ethyl butyrate were purchased from Sigma-Aldrich. Ethyl butyrate and n-butanol were analytical reagents and were dried by
3A
molecular sieves before use. All other chemicals and reagents were of analytical grade. 1-buthyl-3-methyl-imidazolium chloride ([BMIM]Cl) and 1-(1-hydroxyethyl)-3-methyl-imidazolium tetrafluoroborate ([C2OHMIM] [BF4]) were synthesised according to published procedures [11] . [C2OHMIM] [BF4] was checked for the absence of chloride and acid. The IL was passed through a neutral alumina column, dried at 50˚C under reduced pressure for more than 18 h, and stored under dry N2.
The 1H NMR and 13C NMR spectra were obtained on a Brüker AV-400 Fourier transform NMR spectrometer. 1H NMR spectra were referenced to tetramethylsilane in CDCl3.
2.2. pH Titration Procedure
Titration was carried out using a custom-built autotitrator. Place a magnetic stirring bar in the beaker and set the beaker over a magnetic stirrer. An appropriate amount of EDTA acid (1.0 mmol) was dissolved in 20 mL of water. Immerse the electrodes in the solution and start the stirring. 0.1 M [BMIM] [OH] solution was added by a peristaltic pump (Shanghai Hu Xi analysis instrument factory Co., Ltd., model HL-2) running at 1.0 mL∙min−1. The pH of the mixture was recorded with a pH meter (ORION, model 828) interfaced to a computer.
2.3. Synthesis of EDTA IL Buffer
An aqueous solution of 1-buthyl-3-methyl-imidazolium hydroxide ([BMIM]OH) was prepared by passing [BMIM]Cl solution through a column filled with anion exchange resin, as described in the literature [2] [11] . The aqueous [BMIM]OH solution was then neutralized with EDTA acid in a beaker and the pH of the solution was adjusted to 3.90, 6.50, 8.50 or 9.80. The solution was evaporated at 50˚C under reduced pressure to give a viscous liquid, which was then vacuum dried at 50˚C for 18 h to afford EDTA IL buffer. NMR spectra of EDTA IL buffer: pH 9.80 1H NMR (400 MHz, D2O) δ: 7.368 (s, 4H, im-H), 7.320 (s, 4H, im-H), 4.088 (t, 8H, -CH2), 3.784 (s, 12H, im-CH3), 3.447 (s, 8H, N-CH2-COO-), 3.033 (s, 4H, N-CH2-CH2-N), 1.721 - 1.758 (m, 8H, -CH2), 1.199 - 1.217 (m, 8H, -CH2), 0.794 - 0.831 (t, 12H, -CH3);
13C
NMR (400MHz, D2O) δ: 175.703, 135.599, 123.385, 122.119, 57.238, 51.069, 49.200, 35.547, 31.212, 18.706, 12.600. pH 3.90 1H NMR (400 MHz, D2O) δ: 8.067 (s, 2H, im-H), 7.368 (s, 2H, im-H), 7.328 (s, 2H, im-H), 4.085 (t, 4H, -CH2), 3.796 (s, 6H, im-CH3), 3.782 (s, 8H, N-CH2-COO-), 3.580 (s, 4H, N-CH2-CH2-N), 1.717 - 1.754 (m, 4H, -CH2), 1.195 - 1.214 (m, 4H, -CH2), 0.790 - 0.827 (t, 6H, -CH3);
13C
NMR (400 MHz, D2O) δ: 170.500, 135.822, 123.4495, 122.169, 57.939, 51.569, 49.232, 35.602, 31.235, 18.721, 12.624.
2.4. General Procedures of Enzymatic Transesterification in IL
CALB (1.2 mg) was dissolved in 500 μL of [C2OHMIM][BF4] (30 mg of EDTA IL buffer was added in the case of buffered medium) in a 5 mL flask. 110 μL (0.83 mmol) ethyl butyrate and 110 μL (1.21 mmol) n-butanol and 50 μL nonane (internal standard) were added. The reaction mixture was stirred at 40˚C in oil bath for 3 h. After the reaction was complete, the products was decanted from [C2OHMIM][BF4]. The organic phase was analyzed with a gas chromatograph equipped with an FID and a capillary column (SE-30,
30 m
×
0.32 mm
× 0.25 μm). The residual reactant mixture in IL phase was removed in vacuum at 40˚C for more than 1 h. The new cycle was restarted by addition of fresh substrate.
2.5. HRP activity in Mercury Containing Aqueous System
HRP activity was measured by following the H2O2-dependent oxidation of guaiacol at 470 nm. Guaiacol stock solutions (1.0 mM) were prepared by dissolving guaiacol in 0.1 M buffer. For assays done in the presence of Hg2+ ions, appropriate amounts of Hg(NO3)2 stock solution were mixed with 0.1 M buffer and the pH was readjusted whenever required. H2O2 stock solutions (300 mM) were prepared daily by appropriate dilution of 30% H2O2 in distilled water. HRP solutions (10 μg/ml) were prepared by dissolving the enzyme in distilled water. The assay was performed by mixing 500 μL guaiacol stock solutions with 15 μL of HRP solution (final concentration 7.8 nM) and the mixture was then incubated at 298 K for 30 min. The reaction was started by adding 15 μL of 300 mM H2O2. The initial velocity (v0) of the oxidation of guaiacol was determined from linear plotting of the absorbance versus time, using an extinction coefficient of 2.66 ×
10
4 M
−1∙cm−1 for guaiacol-derived oxidation product.
All assays were carried out at 298 K using a UV-Vis spectrophotometer (Unico, UV2800) which cell was connected to a thermostat.
3. Results and Discussion
3.1. Titration Profiles of EDTA
The titration profile of EDTA with [BMIM]OH in water expressed 3 buffering-like regions, which are centered at pH 3.90, 6.50 and 8.50, respectively (Figure 1).
Thus, we considered that it is possible to synthesize new ILs of buffering ability by neutralization of aqueous solutions of [BMIM]OH with aqueous solutions of EDTA at pH 3.90, 6.50 and 8.50, as illustrated in Scheme 1.
3.2. Synthesis and Characterization of EDTA IL Buffer
The structure of EDTA IL buffers were shown in Scheme 1. The IL buffers were synthesized as previously reported [2] [11] and characterized by NMR. The characterization data of EDTA IL at pH 3.90 and pH 9.80 are consistent with the expected compositions and structures. The pH, the dilution value, and buffer values of EDTA IL buffers were summarized in Table 1. It can be seen that the ILs synthesized possess buffering ability and can be used for controlling the ionization of enzymes in both aqueous and non-aqueous media.
![]()
Figure 1. Titration for 0.05 mol∙L−1 EDTA versus 0.1 mol∙L−1 [BMIM]OH in water at room temperature. Titration rate is 1.0 mL∙min−1.
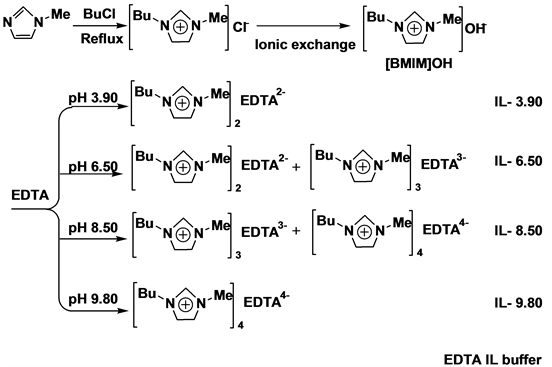
Scheme 1. Synthesis of EDTA IL buffer.
![]()
Table 1. pH, buffer values, and dilution values of the aqueous solution of EDTA IL buffers.
The structure of EDTA IL buffers,
were shown in Scheme 1. The parameters were measured at room temperature. aDilution value is defined as the change of pH on dilution with an equal volume of water. bThe buffer value is defined as the number of moles of strong base required to change the pH of one liter of solution by one unit.
3.3. Enzymatic Transesterification in the Presence of EDTA IL Buffer
Lipase-mediated transesterification is one of the economically viable clean technology for flavor ester production [29] . To test the activity of CALB lipase in dissolved form in ILs, we examined the CALB-catalyzed transesterification of ethyl butyrate with n-butanol. All the reactions were performed under the same conditions, 40˚C and 300 rpm, in this study to eliminate any temperature or mixing effects. The results showed that CALB afforded lower substrate conversion in pure [C2OHMIM][BF4] (8.7%). In contrast, CALB exhibited great transesterification activity in the buffered [C2OHMIM][BF4] (see Figure 2), indicating that the buffer was responsible for the rate enhancement.
The above results showed that the lipase activity is greatly affected by the IL and IL buffer. This is because the ionization constant of ionizable groups of the lipase is greatly affected by the solvent [30] . Thomazeau et al. reported that the acid HNTf2 is more acidic in [BMIM][NTf2] and [BMIM][BF4] than in water
![]()
Figure 2. Transesterification of ethyl butyrate with 1-butanol catalyzed by CALB in buffered ILs. Reaction conditions: CALB powder 1.2 mg; 500 μL of [C2OHMIM][BF4]; 30 mg of EDTA IL buffer; 110 μL of ethyl butyrate (0.83 mmol); 110 μL of 1-butanol (1.21 mmol); 50 μL nonane (internal standard); stirring speed = 300 rpm; temperature = 40˚C. The conversion was calculated by ethyl butyrate.
[31] . Thus, when CALB transfer from water to [C2OHMIM][BF4], the ionization state of the lipase will be changed, resulting in decrease of lipase activity. And addition of EDTA IL buffer can regulate the ionization state of ionizable groups of the lipase to the appropriate state. One can conclude that enzyme activity in ILs is also buffer dependent as in aqueous systems.
The recycling ability of the soluble CALB in buffered [C2OHMIM][BF4] under the above reaction conditions is illustrated in Figure 2. After each reaction, the reactant mixture in the upper layer and CALB-IL in the lower layer were separated by decantation. The CALB-IL phase was then evacuated to remove residual reactant mixture and charged for the next reaction without adding any new enzyme, IL, and buffer. The lipase activity dropped significantly during recycling in the presence of EDTA IL buffer of pH 3.90, suggesting that the lipase was gradually denatured in the acidic environment. However, the conversion of ethyl butyrate can maintain 75% of its initial value after 10 runs in the presence of EDTA IL buffer of pH 6.50 or pH 8.50, indicating that the dissolved CALB in the above buffered [C2OHMIM][BF4] is very stable.
3.4. HRP Activity in Mercury Containing Aqueous System
Oxidative stress causes uncontrolled oxidation, resulting in the progressive deterioration and the collapse of organs and systems in the living organisms [32] . Peroxidases are important detoxifying enzymes serving to rid cells of excess
![]()
Figure 3. Hg2+ effect on HRP activity in EDTA IL buffer system. [HRP] = 7.8 nM and [Hg2+] = 6.0 mM. 500 μL guaiacol solution (1.0 mM); 5 μL H2O2 (300 mM ); temperature, 298 K. The initial velocity (v0) of the oxidation of guaiacol was determined from linear plotting of the absorbance versus time, using an extinction coefficient of 2.66 ×
10
4 M
−1∙cm−1 for guaiacol-derived oxidation product. The error bars represent the standard deviation of measurements.
![]()
Figure 4. Comparison of Hg2+ effect on HRP activity in EDTA IL and BTP buffer systems. [HRP] = 7.8 nM and [Hg2+] = 6.0 mM. r0 and rHg are the initial rate in Hg free and Hg containing systems, respectively.
H2O2, Horseradish peroxidase (HRP) is one of the best characterized peroxidases [33] . The effects of Hg2+ on HRP can be easily assayed through activity of H2O2-dependent oxidation, which can be assayed spectrophotometrically at 470 nm using the H2O2-dependent oxidation of guaiacol.
Figure 3 showed that EDTA IL buffers as Hg2+ chelators protected HRP against Hg2+-induced denaturation and precipitation. Higher pH favored the protection, while at lower pH the protection diminished. Because the concentration of the deprotonated conjugate base of EDTA increases with the increase of pH of solution, its subsequent increased binding to Hg2+ causes the protection of HRP against Hg2+-induced denaturation. On the contrary, there is almost no Hg2+ chelators in BTP buffer system, thereby, Hg2+ inhibition occurs. This chelating effect undoubtedly explained the difference in Figure 3 and Figure 4.
4. Conclusion
In summary, ILs based on EDTA were synthesized by neutralization of [BMIM]OH with EDTA acid for the development of buffered enzymatic IL systems and for enzymatic reaction in heavy metal containing aqueous system. In buffered [C2OHMIM][BF4], transesterification activity of CALB is buffer dependent and CALB is stable during recycles. In EDTA IL buffer aqueous system, EDTA as Hg2+ chelators protected HRP against Hg2+-induced denaturation and precipitation. Higher pH favored the protection, while at lower pH the protection diminished. We can conclude that the new ILs possess both buffering and chelating abilities and can be used for enzymatic applications.
Data Availability
The data used to support the findings of this study are included within the article.
Acknowledgements
We acknowledge the financial supports from the Natural Science Foundation of Fujian Province (2017J01635, 2019J01699), the Natural Science Foundation of China (41576085) and Fujian Provincial Key Laboratory of Food Microbiology and Enzyme Engineering (B18097-7).