Preparation, Characterization, and Application of Manganese Oxide Coated Zeolite as Adsorbent for Removal of Copper(II) Ions from Seawater ()
1. Introduction
There has been an increasing ecological and global public health concern associated with environmental contamination by heavy metals as they may pose risks and hazards to humans [1] - [10] because of their toxicity, persistence and bioaccumulation in food chain [11] [12] [13] . It is normally encountered in industrial wastewater of many industries such as electrical, electroplating, paper manufacturing, pesticides, herbicides and tannery industries. The continuous intake of copper by human beings leads to necrotic changes in the liver and kidney, mucosal irritation; wide-spread capillary damage, depression, weakness, lethargy, anorexia, gastrointestinal irritation and lung cancer [14] [15] [16] . Various methods are used for extraction of heavy metals from aqueous solution, such as reverse osmosis, chemical precipitation, ion exchange, coagulants and flocculation and adsorption [17] [18] [19] [20] . Most of these methods do not seem to be economically feasible because of their relatively high cost. However, the adsorption technique by solid adsorbents is one of the most efficient methods for the wastewater treatment, which exhibits advantages more than the other methods because of simple design and low initial cost [21] . Cellulose is the most abundant organic polymer, environmental-friendly material and has low cost. However, it has low efficiency toward adsorption of heavy metals [20] . One of the cellulose modifications is grafting with certain monomer [22] - [27] . Formation of composite with nano-metal oxides is also another method to improve cellulose efficiency. Iron and manganese oxides nano-particals were mixed with carboxymethyl cellulose [28] , also alumina improved properties of cellulose acetate membrane [29] . Titanium oxide nano-particales were added into cellulose acetate membrane which can lead to improvement of membrane porosity, stability and performance [30] . Cellulose-nanoscale-manganese oxide composite was in situ prepared to remove Pb(II) from water [31] .
In the present work, cellulose is isolated from mangrove trees (Avicennia marina) in Safaga. The extracted cellulose was acetylated and modified by forming composite with NMO followed by grafting copolymerization with acrylamide. The grafted cellulose acetate copolymer—nano manganese oxide composite was functionalized with ethylenediamine, diethylenetriamine, triethylenetetramine and tetraethylenepentanene for higher metal loading. The adsorption characteristics of the novel cellulose acetate composite towards Cu(II) were studied. Finally, the functionalized composite was successfully employed in the recovery of the mentioned metal ion from real wastewater samples.
2. Material and Methods
Chemicals
Acrylamide (C3H5NO, MW = 71.08), tetraethylenepentamine (C8H23N5, MW = 189.3) and acetic anhydride were purchased from sigma. Ethylenediamine (C2H8N2, MW = 60.1) was purcused from Loba Chemie. Diethylenetriamine (C4H13N3, MW = 103.2) was purchased from Alph Aeser. Triethylenetetramine (C6H18N4, MW = 146.2) was purchased from BDH, UK. Potassium permanganate (KMnO4, MW = 158.03, with assay = 99.1%) and copper chloride (CuCl2·2H2O, FW = 170.48, with assay = 98.0%) were purchased from United Company, Egypt. Disodium salt ethylenediamine tetraacetate dehydrate (EDTA) (C10H14N2Na2O8·2H2O, MW = 372.24, with assay = 99.0% - 100.0%) was purchased from Riedel-de Haën.
Collecting of mangrove Avicennia marina and Extraction of cellulose from its roots pulp
Mangrove species Avicennia marina was collected from Safaga coast in Egyptian Red sea (26˚44'0''N/33˚56'0''E). The solid impurities were removed by washing with water and the roots were dried in air and manually separated its pulp. Then it was ground in a rotating ball mill. 50.0 g of dried mangrove Avicennia marina roots pulp powder was treated with 8% (v/v) NaOH solution for 2 h at 80˚C. After the treatment, the slurry was washed with warm water and filtered then neutralized using a solution of 2% (v/v) acetic acid [32] . The powder was stirred in 250.0 ml commercial hypochlorite at pH 10 for 1 h, then in 125.0 ml commercial hypochlorite at pH 6 for 1 h, finally in 3.5% H2O2 for 1 h. The bleached cellulose was filtered and washed with distilled water then dried at room temperature [31] .
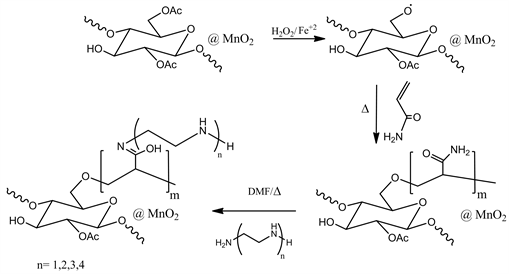
Preparation of cellulose acetate–nano-manganese dioxide hybrid material (CA-NMO)
In the first, cellulose was acetylated by adding 1.5 g of cellulose in a mixture of 7.5 ml of acetic anhydride and 0.015 mL of sulphuric acid 98 wt% (used as a catalyst) and vigorously stirred at 30˚C for 1 hour. The mixture was poured into a large volume of water, and the precipitate was formed. It was filtered through Buckner funnel, washed to neutrality and dried in air.28 Secondly, cellulose acetate–manganese dioxide composites were prepared by impregnating 1.0 g CA into 500.0 ml KMnO4 solution (1.0 g/500.0ml distilled water) for 15 min. KMnO4 impregnated cellulose acetate was subsequently reduced by adding 10.0 ml ethanol drop by drop under stirring till color change to brown. The reaction mixture was kept at rest for two hours. The composites were filtered then washed with distilled water and dried at 60˚C [31] (Scheme 1).
Scheme 1. Preparation of immobilized cellulose acetate composite analogs.
Grafting copolymerization of cellulose acetate-manganese dioxide composites (g-CA-NMO)
2.0 g of CA-NMO were mixed with 8.0 ml of ferrous ammonium sulfate (0.02 M) which transferred into a reaction flask equipped with stirring. The suspension was allowed to stir for 15 min at 30˚C. To the mixture 12 ml of H2O2 (0.2 M) were added with stirring 5 min at the same temperature. The polymerization was started by adding 5.0 g acrylamide. The reaction mixture was stirred at 80˚C for 4 h. Graft copolymer (g-CA-NMO) was recovered by filtration, washed with hot water and finally dried at room temperature [30] .
Immobilization of (g-CA-NMO) with ethylenediamine
The modified cellulose acetate obtained in the above step g-CA-NMO was immobilized by ethylenediamine when 2.0 g of g-CA-NMO was suspended in 1.5 ml of amine and 12.0 ml DMF. The reaction mixture was heated up to 75˚C - 80˚C for 10 h on a water bath [32] [33] . The product obtained was filtered off, washed with methanol, dried in air and referred as (g-CA-EN-NMO).
Immobilization of (g-CA-NMO) with diethylenetriamine
The modified cellulose acetate obtained in the above step g-CA-NMO was immobilized by diethylenetriamine when 2.0 g of g-CA-NMO was suspended in 2.2 ml of amine and 12.0 ml DMF. The reaction mixture was heated up to 75˚C - 80˚C for 24 h on a water bath [33] [34] [35] . The product obtained was filtered off, washed with methanol, dried in air and referred as (g-CA-DETA-NMO).
Immobilization of (g-CA-NMO) with triethylenetetramine
The modified cellulose acetate obtained in the above step g-CA-NMO was immobilized by triaethylenetetramine when 2.0 g of g-CA-NMO was suspended in 3.0 ml of amine and 12.0 ml DMF. The reaction mixture was heated up to 75˚C - 80˚C for 24 h on a water bath [33] [34] [35] . The product obtained was filtered off, washed with methanol, dried in air and referred as (g-CA-TETA-NMO).
Immobilization of (g-CA-NMO) with tetraethylenepentamine
The modified cellulose acetate obtained in the above step g-CA-NMO was immobilized by tetraethylenepentamine when 2.0 g of g-CA-NMO was suspended in 5.0 ml of amine and 12.0 ml DMF. The reaction mixture was heated up to 75˚C - 80˚C for 72 h on a water bath [33] [34] [35] . The product obtained was filtered off, washed with methanol, dried in air and referred as (g-CA-TEPA-NMO).
Adsorption studies
All batch sorption experiments were carried out at room temperature (25˚C ± 1˚C). Each experiment was performed in 50 mL volumetric flask by mixing a 1.0 ml of 0.1 mol·l−1 metal ion solution with 100 ± 1 mg of the dry sorbent and the pH was adjusted by adding 9.0 ml of buffer solutions (pH 1.0 - 7.0). This mixture was shaken by using an automatic shaker for 30 min then filtered and washed with 50 ml of distilled water. The residual metal ion content in the filtrate was determined and each experiment was repeated three times. The previously mentioned batch experiment procedure was carried out at different shaking time of 1, 5, 10, 15, 20, 25, 30, 35, 40, 45, 50, 55 and 60 min and optimum buffering condition (pH 7.0).
In each batch experiment, the sorption metal capacity value was calculated from a metal mass balance (Equation (1)).
(1)
where, Co and C (mol·l−1) are the initial and residual metal ion concentration respectively, V (l) is the volume of the solution, m (g) is the mass of dry sorbent and q (mmol·g−1) is the sorption metal capacity.
Similar batch experiments were carried out by mixing a 1.0 mL of 0.1 mol·l−1 metal ion solutions with 9.0 ml of buffer solution (pH 7.0) and by using different sorbent masses of 5.0, 10.0, 20.0, 40.0, 60.0, 80.0, 100.0, 200.0 and 250.0 mg. The sorption mixture was shaken for 30 min and the percentages of metal removal were then determined in the residual metal ion content in the filtrate.
Sorption equilibrium and isotherm studies were evaluated by varying the initial metal ion volume. A 0.1 M solution of 0.6, 0.8, 1.0, 1.2, 1.4, and 1.6 ml metal ion was used. This solution was mixed with 9.0 mL of buffer pH 7.0 and 100 ± 1 mg of the dry sorbent and shaken for 30 min. The mixture was filtered and washed with 50 ml of distilled water.
Extraction of Cu(II) ion from real hard water samples
Three different water samples were examined for removal of Cu (II) by the sorbents. The first sample is drinking tap water, the second was obtained from Mediterranean seawater at Alexandria, Egypt and the third is industrial wastewater sample was collected from Al-Nubariya canal. These water samples were spiked with 1 ppm of the selected metal ion. A 1.0 l of water sample was passed over a micro-column system packed with 100 ± 1.0 mg of sorbent by using a flow rate of 10 ml·min−1 under air pressure [35] . Effluent solution was collected and subjected to metal determination by atomic absorption analysis. This procedure was repeated three times.
Instrumentations
FT-IR spectra were recorded on a Burker Tensor 37 FTIR between 4000 and 400 cm−1. Thermogravimetric analyses (TGA) were caried out in a nitrogen atmosphere using Perkin–Elmer TGA7 Thermobalance. The flow rate of N2 was adjusted at 20 ml/min and heating rat was 10˚C /min. Scanning electron microscope (SEM) was performed using FEI Quanta 250. SHIMADZU atomic absorption spectrophotometer (AAS) AA-6800 was used to determine metal ions concentrations.
3. Results and Discussion
Characterization and surface morphology
FT-IR spectra of cellulose, CA, g-CA-NMO, g-CA-EN-NMO, g-CADETA-NMO, g-CA-TETA-NMO and g-CA-TEPA-NMO are shown in (Figure 1). There are two differences between the spectrum of cellulose and CA (Figure 1 curve a, b, respectively) which proves acetylation of extracted cellulose, the first one is the decrease in intensity of OH peak at 3444 cm−1 in CA compared to that of cellulose which was at 3407 cm−1. The second evidence is the presence of three important ester bands at 1752 cm−1 (C = O ester), 1378 cm−1 (C-H bond in −O(C = O)-CH3 group) and 1241 cm−1 (−CO-stretching of acetyl group) in CA. For FT-IR spectra of g-CA-NMO, g-CA-EN-NMO, g-CA-DETA-NMO, g-CA-TETA-NMO and g-CA-TEPA-NMO (Figure 1 curve c, d, e, f, g, respectively), the broad bands of hydrogen bonding of OH groups are shown at 3420, 3400, 3410, 3420 and 3383 cm−1, respectively, but the N-H band in g-CA-EN-NMO, g-CA-DETA-NMO, g-CA-TETA-NMO and g-CA-TEPA-NMO cannot be detected due to its overlapping with the broad OH band.
The spectrum of g-CA-NMO (Figure 1 curve c) exhibits a little shift in C=O peak compared to that of in CA spectrum from 1752 cm−1 to 1747 cm−1 due to vibrational modes of C=O after grafting. For FT-IR spectra of g-CA-EN-NMO, g-CA-DETA-NMO, g-CA-TETA-NMO and g-CA-TEPA-NMO (Figure 1 curve d, e, f, g, respectively), C=N bands are detected at 1654, 1656, 1655 and 1651 cm−1, respectively and C-N stretching bands are detected at 1051, 1058, 1059 and 1064 cm−1, respectively. The bands at 507, 509, 511, 497 and 522 cm−1 are assigned to Mn-O stretching vibrational modes in g-CA-NMO, g-CA-EN-NMO, g-CA-DETA-NMO, g-CA-TETA-NMO and g-CA-TEPA-NMO, respectively. The presence of NMO in the g-CA-NMO, g-CA-EN-NMO, g-CA-DETA-NMO, g-CA-TETA-NMO and g-CA-TEPA-NMO has negligible effects on their spectra bands (29). In addition, the C-H stretching bands at 2907, 2928, 2928, 2960, 2964, 2924 and 2929 cm−1 and scissoring motion of CH2 bending vibration at 1430, 1435, 1432, 1400, 1410, 1439 and 1464 cm−1 are characterized for cellulose, CA, g-CA-NMO, g-CA-EN-NMO, g-CA-DETA-NMO, g-CA-TETA-NMO and g-CA-TEPA-NMO, respectively, where, β-glycosidic linkage in cellulose spectrum was observed at 897 cm−1.
![]()
Figure 1. FT-IR spectra of (a) Extracted cellulose, (b) CA, (c) g-CA-NMO, (d) g-CA-EN-NMO, (e) g-CA-DETA-NMO, (f) g-CA-TETA-NMO and (g) g-CA-TEPA-NMO.
TGA analysis for g-CA-EN-NMO, g-CA-DETA-NMO, g-CA-TETA-NMO and g-CA-TEPA-NMO (Figure 2 curve b, c, d, e, respectively) exhibit firstly weight loss which related to the physically and chemically volatilization and desorption of water molecule 22.9% within temperature range 22˚C - 183˚C, 23.7% within temperature range 26˚C - 189˚C, 39.9% within temperature range 20˚C - 163˚C and 22% within temperature range 28˚C - 141˚C, respectively, then decomposition of immobilized grafted cellulose acetate 36.9% within temperature range 183˚C - 338˚C, 42.1% within temperature range 189˚C - 433˚C, 19.4% within temperature range 163˚C - 425˚C and 58.4% within temperature range 141˚C - 799˚C, respectively, finally constancy refers to the high thermal stability of NMO.
The SEM images of CA, CA-NMO, g-CA-NMO, g-CA-EN-NMO, g-CA-DETA-NMO, g-CA-TETA-NMO and g-CA-TEPA-NMO are shown in (Figure 3). SEM images indicate surface morphology of CA upon treatment with NMO loading, grafting copolymerization and immobilization, which clearly showing the presence of similar shiny bulky particles loaded with NMO on its irregular surfaces and indicate homogeneous distribution between them.
Adsorption studies
Effect of pH
pH effect is the most important factor which controls the adsorption process due to its influence on the adsorbent surface, the solution chemistry and the heavy metals. The effect of pH on the adsorption of Cu(II) on CA, CA-NMO, g-CA-EN-NMO, g-CA-DETA-NMO, g-CA-TETA-NMO and g-CA-TEPA-NMO at 25˚C mainly depends on the surface charge of the adsorbents as well as their functional groups (Figure 4). The adsorption capacity is gradually increased as the solution pH increased and reached their maximum values at pH 7 [36] [37] . At low pH values (1-5), the surface of adsorbents would be surrounded by high concentration of hydronium ions and the functional groups responsible for binding with heavy metal were protonated, so this decrease interaction of the metal ions with binding sites of the adsorbents. As the pH increased, the overall surface on the adsorbents became negative and adsorbents functional groups are deprotonated, so adsorption increased. At pH values above pH 5.0, the metals adsorption is indicated by two mechanisms. The first one is the complexation and/or ion-exchange of the metal ions by the deprotonated hydroxyl, amine groups in sorbents. The second mechanism is the adsorption of the metal ions by nano-manganese dioxide in CA-NMO, g-CA-EN-NMO, g-CA-DETA-NMO, g-CA-TETA-NMO and g-CA-TEPA-NMO (17, 18, 25).
![]()
Figure 4. Effect of pH on the removal of Cu(II).
Effect of contact time
The effect of selected shaking time values (1, 5, 10, 15, 20, 25, 30, 40, 50 and 60 min.) on adsorption of Cu(II) by CA, CA-NMO, g-CA-EN-NMO, g-CA-DETA-NMO, g-CA-TETA-NMO and g-CA-TEPA-NMO is represented in (Figure 5). The metal capacity values of sorbents for metal ion gradually increased with the increase in shaking time from 1 - 30 min, where the complete extraction of Cu(II) ion was obtained above 30 min. The adsorption process becomes less efficient after 30 min due to complete saturation of the adsorbent surface with the target metal ion. The removal of Cu(II) ions by CA, CA-NMO, g-CA-EN-NMO, g-CA-DETA-NMO, g-CA-TETA-NMO and g-CA-TEPA-NMO was found to proceed via one adsorption step (17, 18).
Effect of sorbent dosage
The removal of Cu(II) was studied by varying the sorbent dose of different sorbents (5 - 250 mg) in aqueous system at pH = 7 and 25˚C as shown in (Figure 6). Increasing sorbent mass the surface area increase as well as greater number of active binding sites and the percentages of extracted Cu(II) ions increase [37] . The percentage removal of Cu(II) by different sorbents were found to increase with increasing sorbent dose. The percentage removal is increased from 18.4% to 37.8% in case of CA, 14.9% to 57.4% for CA-NMO, 9.8% to 74.5% for g-CA-EN-NMO, 11.8% to 76.5% for g-CA-DETA-NMO, 26.7% to 95% for g-CA-TETA-NMO and 29.4 to 95.1 for g-CA-TEPA-NMO, as the adsorbent dose is increased from 5 - 100 mg. This behavior is due to the availability of higher binding sites and greater surface area for removal of Cu(II) metal ion with increasing the sorbent dosage (17, 18). The removal of Cu(II) had small increase in higher adsorbent dosage (above 100 mg).
Adsorption isotherm
The adsorption isotherm is a dynamic balance describing the equilibrium process between metal ions and solid surface of an adsorbent. Adsorption isotherm higher q max was observed for larger ionic radii ions [38] . The isotherm data for the adsorption of Cu(II) ion onto CA, g-CA-EN-NMO, g-CA-DETA-NMO, g-CA-TETA-NMO and g-CA-TEPA-NMO adsorbents was accomplished by fitting to Langmuir and Freundlich models. The Langmuir isotherm and its linearized form, that assume a reversible sorption process on the homogeneous surface forming a monolayer sorption without interactions between the adsorbed species, are represented by (Equations (2) (3)).
(2)
(3)
where qe (mg·g−1) is the equilibrium concentration of metal ions on the adsorbent surface, qmax (mg·g−1) is the maximum adsorption capacity, Ce (mg·L−1) is the liquid phase equilibrium concentration of metal ions and b (L·mg−1) is the affinity constant.
The Freundlich isotherm and its linearized form are represented by (Equations (4) (5)). This model is an empirical equation that assumes sorption on a heterogeneous energetic distribution of active binding sites.
(4)
(5)
where Kf (L·g−1) is the sorption capacity constant and n is the sorption intensity constant.
![]()
Figure 5. Effect of contact time on the removal of Cu(II).
![]()
Figure 6. Effect of adsorbent dose on the removal of Cu(II).
These two isotherm models were investigated for the removal of Cu(II) by using different initial metal ion volume for 30 min contact time and 100.0 mg sorbent at pH 7 and at constant temperature 25˚C ± 1˚C. The linear graphical representations of the Langmuir model for Cu(II) sorption by CA, g-CAEN-NMO, g-CA-DETA-NMO, g-CA-TETA-NMO and g-CA-TEPA-NMO are represented in (Figure 7). The identified isotherm constants and capacity parameters, for Cu(II) are listed in (Table 1). The values of linear regression coefficients (R2) indicate that the Cu(II) sorption processes by all sorbents are more fitted to Langmuir isotherm rather than Freundlich isotherm, which indicates that chemisorption of metals is obtained by these sorbents (17,18).
Analytical applications of g-CA-EN-NMO, g-CA-DETA-NMO, g-CATETA-NMO and g-CA-TEPA-NMO for removal of Cu(II) ion from real water samples
Multi-stages micro-column mode was employed for removal of Cu(II) ion from tap, sea and industrial wastewater samples to evaluate the validity of the uses of the modified cellulose acetate sorbents g-CA-EN-NMO, g-CA-DETANMO, g-CA-TETA-NMO and g-CA-TEPA-NMO. The percentages extraction of Cu(II) from the real samples by these sorbents was collected in (Table 2). The removal percentage of Cu(II) ion found to be 82.9% - 91.5% for g-CA-EN-NMO, 83.5% - 92.9% for g-CA-DETA-NMO, 90.2% - 96.7% for g-CA-TETA-NMO and 93% - 100% for g-CA-TEPA-NMO. This is an indication on higher efficiency of sorbents towards trace Cu(II) metal ions. This reveals that the modified materials have better Cu(II) adsorption ability and offers valuable data for environmental managers and policymakers for future applications
4. Conclusion
The present work affords a number of environmental friendly cellulose adsorbents for the remediation of Cu(II) ion. The modified cellulose adsorbents are all
![]()
Figure 7. Langmuir isotherm for Cu(II) by CA, g-CA-EN-NMO, g-CA-DETA-NMO, g-CA-TETA-NMO and g-CA-TEPA-NMO.
![]()
Table 1. Langmuir and Freundlich isotherm constants for Cu(II) sorption.
![]()
Table 2. Percentages extraction of Cu(II) from real samples by g-CA-EN-NMO, g-CA-DETA-NMO, g-CA-TETA-NMO and g-CA-TEPA-NMO sorbents.
characterized by higher capacity toward metal ions extraction in presence of buffering condition. Extracted cellulose was converted to CA then formed composite with NMO. The composite was surface modified with acrylamide using Fenton’s reagent (H2O2/FeSO4) then immobilized by Ethylenediamine, diethylenetriamine, triethylenetetramine and tetraethylenepentanene and was used for adsorption of Cu(II) from water. The sorption efficiency of these different sorbents was investigated at different conditions. The maximum sorption efficiency values were obtained at pH 7. The sorption equilibria were described by Langmuir and Freundlich models. The validity of the uses of these extractors was applied for the removal of Cu(II) from water, seawater and industrial wastewater.