Possible Effects of Location and Handling on Some Fungi Associated with Rotting Citrullus lanatus Thunb. (Watermelon) ()
1. Introduction
Citrullus lanatus (watermelon) is a tropical fruit which can be found in most parts of Africa, Asia, United States and Russia [1] . According to [2] , they originated in Africa and had been in cultivation for more than 4000 years in the drier parts of the continent and throughout India and parts of Asia. The plant is well adapted to tropical and subtropical regions where cultivation is high. Its existence started in the dry regions of southern Africa and it is produced all over the world particularly in the semi-arid regions [3] . They are among the most widely grown vegetable crops in the warmer parts of the world [3] [4] [5] .
In Nigeria, the largest production of watermelon (Citrullus lanatus Thunb family cucurbitaceae) comes from the Northern part in Nigeria [6] . They are grown and are quite popular in the Northern part of Nigeria especially in the central and north-eastern part [7] .
It is highly nutritious and low in calories [8] and can be used as an appetizer or snack [9] . It’s nutritional and medicinal values have been well documented [7] . Apart from being a source of some vitamins, it is known to be popular for its thirst quenching ability in Africa during water shortage [4] [10] [11] . It is a natural and rich source of phytochemical compounds which are believed to be beneficial for human health and well-being [12] . It is known to play a role in the protection of prostrate and oral cancer [7] . The seeds are used in the treatment of hypertension, diabetes, diarrhea and gonorrhea [13] . It is a fruit that is a good source of potassium which is an important component of cell and body fluids that helps in controlling heart rate and blood pressure thereby preventing stroke and coronary heart diseases [14] . It contains high level of antioxidant which decreases the risk of kidney stone and bone loss due to old age [15] .
However, apart from insect infestation, watermelon is susceptible to several plant pathogens (fungi, bacteria, nematodes and viruses) which attack the roots, foliage and fruit thereby limiting their marketability and the yield of the growers [16] [17] . The aim of the study therefore is to examine the probable effect of location and handling on fungi associated with rotting watermelon.
2. Methodology
2.1. Collection of Samples
Rotting watermelon samples were collected from 2 vendors each from 5 different markets in Ibadan, Oyo state viz., Sango, Bodija, Ojoo, Eleyele, Mokola. All the samples were labelled appropriately and taken to the Pathology Laboratory, Department of Botany, University of Ibadan.
2.2. Isolation and Identification of Fungi from Rotting Watermelon
Small portions were excised from different parts of the rotting watermelon using a sterile knife and cultured on Petri Plates of acidified Potato Dextrose Agar (APDA). The Petri Plates were incubated at room temperature (27˚C) for 5 days when they were observed daily for fungal growth. All experiments were done in duplicates and each isolated fungus was later sub-cultured unto sterile Plates of APDA to obtain pure cultures which were later identified. Pathogenicity test was later carried out for the isolated fungi.
2.3. Data Collection and Statistical Analysis
The isolation of each fungus from all the samples was recorded as either present (1) or absent (0). The data collected were subjected to analysis of variance (ANOVA) using the Generalized Linear Model option of SAS 9.3 version.
3. Results
Three fungi were isolated from the rotting Citrullus lanatus (watermelon) fruits viz., Aspergillus flavus (Plate 1), Aspergillus niger (Plate 2), and Saccharomyces cerevisae.
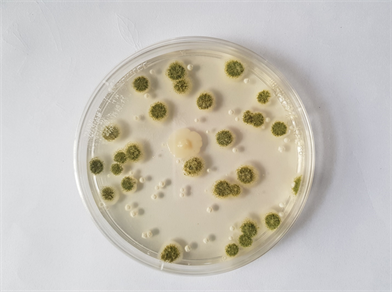
Plate 1. Aspergillus flavus from rotting watermelon obtained from Bodija, Mokola and Sango market in Ibadan.

Plate 2. Aspergillus niger from rotting watermelon obtained from Bodija, Mokola and Ojoo markets in Ibadan.
Table 1 shows the means comparison of the isolated fungi in the watermelon obtained from the five different markets in Ibadan. The incidence of Aspergillus niger in rotting Citrullus lanatus obtained from Bodija, Mokola and Ojoo markets was significantly (p ≤ 0.05) higher than those in Citrullus lanatus obtained from the other two markets. The incidence of Aspergillus flavus in rotting Citrullus lanatus obtained from Bodija, Sango and Mokola markets was significantly (p ≤ 0.05) higher than those in Citrullus lanatus obtained from the other two markets. However the incidence of Saccharomyces cerevisae in rotting Citrullus lanatus obtained from Bodija and Mokola markets was significantly (p ≤ 0.05) higher than those in Citrullus lanatus obtained from the other markets.
Table 2 shows the overall means comparisons of incidence of the isolated fungi in Citrullus lanatus obtained from the five markets in Ibadan. Generally, the incidences of all the isolated fungi in the rotting Citrullus lanatus obtained from Bodija and Mokola markets were significantly (p ≤ 0.05) higher than those in Citrullus lanatus obtained from Sango and Ojoo markets. The incidences of all the isolated fungi in the rotting Citrullus lanatus obtained from Sango and Ojoo markets were significantly (p ≤ 0.05) higher than those in Citrullus lanatus obtained from Eleyele market.
Table 3 shows the means comparison of the isolated fungi in Citrullus lanatus obtained from the two vendors in each of the markets. The incidences of the three isolated fungi in the rotting Citrullus lanatus obtained from the two vendors in each of the markets were not significantly (p ≤ 0.05) different. Similar result was obtained in Table 4 where overall incidence of the isolated fungi in Citrullus lanatus from the two vendors in each of the markets were compared. The ANOVA table for incidence of the isolated fungi in Citrullus lanatus obtained from the five markets is given in Table 5. The F-values (p > 0.0001) for model and location were all highly significant for the three isolated fungi.
![]()
Table 1. Means comparison of the isolated fungi in Citrullus lanatus obtained from five different markets in Ibadan, Nigeria.
Means with the same letters are not significantly different (p ≤ 0.05).
![]()
Table 2. Overall means comparisons of incidence of the isolated fungi in Citrullus lanatus obtained from the five markets in Ibadan, Nigeria.
Means with the same letters are not significantly different (p ≤ 0.05).
![]()
Table 3. Means comparison of the isolated fungi in Citrullus lanatus obtained from the two vendors in each of the markets.
Means with the same letters are not significantly different (p ≤ 0.05).
![]()
Table 4. Overall means comparisons of incidence of the isolated fungi in Citrullus lanatus obtained from the two vendors in each market.
Means with the same letters are not significantly different (p ≤ 0.05).
![]()
Table 5. ANOVA table for incidence of the isolated fungi in Citrullus lanatus obtained from five markets in Ibadan, Nigeria.
*Significant.
4. Discussion
The significant occurrence of all the isolated fungi in watermelon obtained from one market compared to the other might be as a result of infection from the fields where the watermelon were harvested. Postharvest pathogens which come as a result of infection from the field is a common occurrence. S. cerevisae are known to be saprophytes growing on any substrates containing sugar [18] and they possibly originate from the field and are transported on surfaces of fruits to fruit stalls and stores. Saccharomyces cerevisae has also been reported to be associated with spoilage of tomato, pepper and orange [19] [20] [21] [22] . However, Nwachukwu et al. [23] [24] [25] [26] found S. cerevisiae as contaminants of fresh sliced ready-to-eat watermelon fruits sold on the streets of some cities in Nigeria.
A. niger and A. flavus has been isolated from watermelon in storage and deterioration of fruits by A. niger has been reported in watermelon by some researchers including [27] [28] . It has also been reported in other fruits by other researchers such as [29] [30] [31] [32] .
However, the significant occurrence of the three fungi in watermelon obtained from Bodija and Mokola compared to other markets is suggestive. This might be due to the fact that the different locations of the markets play a part in determining occurrence of fungi in the watermelon. It is suggesting a strong association between these fungi and the two markets (Bodija and Mokola). It might as well be indicative of the hygiene conditions of these two markets. This is also corroborated by the significant occurrence of the isolated fungi in watermelon from Sango and Ojoo markets compared to those from Eleyele market. It might mean that fungal occurrence on watermelon might not be only as a result of infection from the field, especially if the fruits from the markets are not from a common farm. However results obtained with the vendors suggested that individual vendors of the fruit might not play a part in determining occurrence of fungi in the fruit. The significant F value (p > 0.0001) for model for the three isolated fungi shows appropriateness of the model. It shows the goodness of fit of the model. The significant F values (p > 0.0001) for location for the three isolated fungi confirm the significant F values for the models. It shows that the location where the watermelon is purchased most likely determines occurrence of the fungi in the fruit. It suggests a strong association of these fungi with Mokola and Bodija markets. The significant F values (p > 0.0001) for location also confirm the results obtained with the isolated fungi in watermelon from the different markets.
5. Conclusion
Saccharomyces cerevisae are known to be saprophytes growing on any substrates containing sugar and they possibly originate from the field and are transported on surfaces of fruits to fruit stalls and stores. Vendors and consumers alike are advised to be mindful of where they purchase their fruits. Consumers particularly need to maintain a high level of hygiene, especially after purchase before consumption.