1. Introduction
A white hard polysaccharide chitin, which known as 2-acetamido-2-deoxy-D-gluco-pyranose units through (1 → 4) linkage, is extracted from the crustacean’s exoskeletons and also from crabs and shrimps [1] [2] . The alkaline deacetylation of chitin produces a very useful material chitosan, which known as a copolymer of (1 → 4) linked 2-amino-2-deoxy-D-gluco-pyranose units, and also it is found naturally in some fungal cell walls. Since it is non-toxic and presents excellent biological properties such as biodegradation in the human body, immunological, antibacterial, and wound-healing activity [3] [4] , as shown in (Scheme 1), chitosan has been widely used in food and pharmaceutical processes and in medical and agricultural drugs [5] [6] [7] [8] [9] . It can be found also in the skeleton of
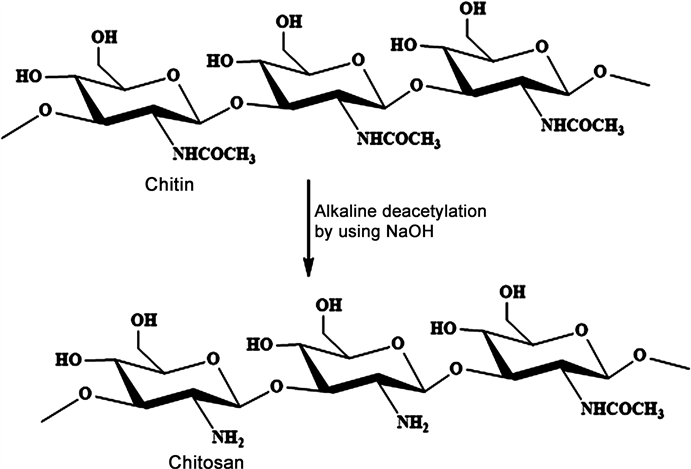
Scheme 1. Chemical structures of chitin and chitosan.
crab, shrimp and lobster, as well as in the exoskeleton of marine zooplankton spp., including coral and jellyfishes [10] . Also, the chitin can be extracted from various sources to be converted to chitosan by different degree of dacetaylation during using different concentration of NaOH [11] . Due to solubility of chitosan in acidic aqueous medium, various applications at industrial area can be found for it; its solubility is due to the degree of acetylation, molecular weight, and distribution of the acetyl and amino groups along the chain. Also, antimicrobial activity is attributed to chitosan when the amino groups are in cationic form, which means that antimicrobial activity of chitosan is higher at low pH [12] . Chitosan has a broad-spectrum antimicrobial activity against both Gram-positive and Gram-negative bacteria [13] .
The aim of the present study is the preparation of low coast chitosan with different degree of deacetylation from wastes of Egyptian shrimp shells to use it as a key material for many applications.
2. Materials and Methods
2.1. Materials
Raw shrimps stated as large size were purchased from Egyptian (Market, Eloubor city, Egypt), Sodium hydroxide (NaOH) (Aldrich, Egypt), Hydrochloric acid (HCl) (Aldrich, Egypt), and acetic acid (Aldrich, Egypt). They were then diluted to the concentration required for the methodology with distilled water. All chemicals were used without further purification.
2.2. Measurements
The infrared spectra were measured on Perkin-Elmer-1430 infrared spectrophoto-meter using the potassium bromide Wafer technique. X-ray diffractograms of polymers were obtained with a Phillips X-ray radiation unit (Generator PW-1390) and Ni-filtered Cu. Thermogravimetric analysis (TGA) was carried out in a nitrogen atmosphere using a Shimadzo TGA-50H. The morphology of the different hydrogels was investigated using JXA 850 prop micro analyzer scanning electron microscope (SEM). The solubility of the polymers was examined using 0.02 g of polymer in 5 ml solvents at room temperature 25˚C.
2.3. Methods (Extraction of Chitosan)
The extraction of chitosan can be carried out by different four methods under different conditions after removing the loose tissue from the shrimp shells then washed, dried and grind to obtain dry powder. The major procedure for obtaining chitosan is based on the alkaline deacetylation of chitin with strong alkaline solution at different period of time.
2.3.1. Extraction
Method 1
1) Deproteinization process
The deproteinization was occurred by heating of 3 gm of shrimp shells powder after adding 2 N NaOH with ratio of 12ml:1g (w/v) at 70˚C for 4 h. The product was neutralized by washing under running tap water. The solid was collected and washed with distilled water. The solid product was dried in vacuum and weighed with analytical balance.
2) Demineralization process
The dry solid was treated with 10% HCl (3.25 N) with ratio of 14ml:1g (w/v) at room temperature and kept for 4 h. The solid product was collected and washed with distilled water. The solid was then dried.
3) Deacetylation
Then the demineralized product was treated with 35% NaOH (8.75 N) with ratio of 14ml:1g (w/v) at room temperature for 75 h. with stirring. The deacetylated solid was filtered then collected and washed with distilled water. The deacetylated product was dried in a vacuum to give 1.51 gm and then labeled as Cs1.
2.3.2. Extraction
Method 2
1) Demineralization process
The demineralization was carried out by weight 3 gm of shrimp shells powder by using 4% HCl (1.3 N) with ratio of 14ml:1g (w/v) at room temperature for 24 h. The product was washed to neutrality under running tap water. The solid was collected and washed with distilled water, then dried in a vacuum.
2) Deproteinization process
Deproteinization was carried out using 5% NaOH (1.25 N) with ratio of 12ml:1g (w/v) at 90˚C for 24 h. The deproteinized product was collected and washed with distilled water.
3) Deacetylation
The product was deacetylated with 70% NaOH (17.5 N) with ratio of 14ml:1g (w/v) at room temperature for 75 h. with stirring. The solid was collected and washed with distilled water. The deacetylated product was then dried in a vacuum, producing 2.04 gm and labeled as Cs2.
2.3.3. Extraction
Method 3
1) Deproteinization process
The deproteinization process was carried out by weight 3 gm of shrimp shells powder by using 5% NaOH (1.25 N) with a weight to volume ratio of 1g:8ml (w/v). The solution with shrimp shells was refluxed at 70˚C for 3 h. The product was collected and washed until clear solution. It was then dried in a vacuum. The product was decolorized with pure acetone for 24 h. The product was collected and washed to neutrality, then dried.
2) Demineralization process
The decolorized product was demineralized by using 1% HCl (0.32 N) with a weight to volume ratio of 1g:10ml for 24 h. at room temperature. The product was collected and washed to give light brown powder.
3) Deacetylation
The N-deacetylation of the demineralized product was carried out by using 55% NaOH (12.5 N) with weight to volume ratio of 1g:5ml at 100˚C for 12 h. The product was washed with distilled water and dried to produce 1.69 gm and then labeled as Cs3.
2.3.4. Extraction
Method 4
1) Demineralization process
Weight 3 gm of shrimp shells powder, then the powder was treated by 1 N NaOH (4%) with weight to volume ratio of 1 g: 10 ml for 24 h. at room temperature. It was washed and dried in vacuum. The solid from the alkaline treatment was then demineralized by using 1 N HCl (3%) with weight to volume ratio of 1g:10ml for 24 h. at room temperature. It was washed and dried in vacuum.
2) Deproteinization process
The demineralized product was deproteinized by using 1 M NaOH (4%) with weight to volume ratio of 1g:10ml for 24 h. at room temperature. It was washed and dried in vacuum. The product from deproteinization was decolorized using pure acetone with for 24 h. at room temperature. It was washed and dried in vacuum.
3) Deacetylation
From decolorization, the product was then deacetylated by using 50% NaOH with weight to volume ratio of 1g:10ml for 24 h. at room temperature. The product was washed and dried in vacuum to produce 1.14 gm and then labeled as Cs4.
3. Result and Discussion
The major procedure for extraction of chitosan from shrimp shells powder, which waste shrimp shells in Egypt, is a preliminary study to evaluate various levels of deacetylated chitin for different applications as: pharmaceutical processes and in medical and agricultural drugs, and the extraction is based on the alkaline deacetylation of chitin with strong alkaline solution via deproteinization, demineralization and deacetylation of shrimp shells powder at different conditions to give the following chitosan samples: Cs1, Cs2, Cs3 and Cs4 respectively.
3.1. Characterization of the Prepared Chitosan
The chitosan samples: Cs1, Cs2, Cs3 and Cs4 were characterized by (FT-IR) to identify the functional groups in chitosan. X-ray diffractometry (XRD) is to analyze the crystallinity of the product; thermogravimetric analysis (TGA) is to study the thermal stability; the elemental analysis is to calculate the degree of deacetylation. Finally, Scanning electron microscope is to demonstrate the morphology of the product.
3.1.1. FTIR Spectroscopy
The IR spectral data for the produced chitosan [Cs1] [Cs2] [Cs3] [and Cs4] revealed the following peaks: peak at 3440.9 cm−1, 3396.1 cm−1, 3438.7 cm−1, 3441.5 cm−1 is assigned to -OH and -NH stretching vibrations, while the peaks at 2960.8 - 2890.4 cm−1, 2971.3 cm−1, 2959.7 - 2890.6 cm−1, 2961.4 - 2890.5 cm−1 are assigned to the aliphatic C-H stretching vibration in the -CH and -CH2 groups. The amide frequencies consist of the -C-O bond stretch of the remaining acetamido groups and the N-H bending vibrations of the -NH2 groups are observed at 1663.5 and 1559.9 cm−1, 1754.1 and 1664.7 cm−1, 1659.5 and 1561.7 cm−1,1656.3 and 1562.5 cm−1 respectively. The peak at 1429.9 cm−1, 1451.1 cm−1, 1418.2 cm−1, 1419.1 cm−1 is assigned to -NH2 deformation. Further bending vibrations are observed at 1379.4 cm−1, 1409.5 cm−1, 1380.5 cm−1, 1381.8 cm−1 for the C-C-H symmetric bending vibration in the alcohol. Stretching vibrations are also observed at 1317.2 and 1156.9 cm−1, 1154.5 cm−1, 1316.0 and 1156.8 cm−1, 1316.9 and 1156.9 cm−1 for the C-N stretching vibration and at 1072.7 and 1032.3 cm−1 for the -CO stretching vibration of the alcohol groups as shown in Figure 1.
3.1.2. X-Ray Diffraction (XRD)
The X-ray diffraction is used in the characterization of crystalline materials. By studying the X-ray diffraction of the extracted chitosan from the four methods, it can be concluded that the order of crystallinity is of different chitosan samples: [Cs2 > Cs3 > Cs1> Cs4], so the highest crystallinity is shown by chitosan produced from method 2 [Cs2] as shown in Figure 2.
3.1.3. Thermal Stability (Thermogravimetric Analysis) (TGA)
The thermograph of the produced chitosans [Cs1] [Cs2] [Cs3] [Cs4] were
![]()
Figure 1. Infrared spectra of (a) Cs1, (b) Cs2, (c) Cs3 and (d) Cs4.
![]()
Figure 2. X-ray diffraction pattern for (a) Cs4, (b) Cs1, (c) Cs3 and (d) Cs2.
evaluated by using TGA in air at heating rate 10˚C/min and recorded in Figure 3 and Table 1: It shows the following data: the weight loss of the extracted chitosan by the four methods at the beginning may be due to the ease of degradation of the amide groups; however the weight loss in the high temperature range is attributed to the degradation of the main chain.
The data reported in Table 1 showed that chitosan (Cs2) possesses the highest thermal stability
3.1.4. Degree of Deacetylation for Chitosan
By using the elemental analysis, the percentage of free amino groups on the chitosan can be determined by using the following equation [14] :
The location of 5.145 is related to completely N-deacetylated chitosan (C6H11O4N repeat unit) and 6.186 is the fully N-acetylated polymer (C8H13O5N repeat unit). The value of degree of deacetylation of chitosan samples was calculated and reported in Table 2. The data indicated that the highest degree of deacetylation (DD) shown by the Cs2, Cs3. It can be concluded that the degree of deacetylation of chitosan increased by increasing the concentration of the NaOH used in. The elemental analysis and the degree of deacetylation are shown in Table 2.
![]()
Figure 3. TGA for (a) Cs1, (b) Cs2, (c) Cs3 and (d) Cs4.
![]()
Table 1. Thermal properties of the extracted chitosan by the four methods.
![]()
Table 2. The elemental analysis, and the degree of deacetylation of chitosan.
3.1.5. Studies of the Morphology
The SEM photographs show the morphologies of the four extracted chitosan as shown in Figures 4-7 which show the morphologies of the extracted chitosan by
![]() (a)
(a) ![]() (b)
(b) ![]() (c)
(c)
Figure 4. SEM of Cs1 at (a) 200 µm. (b) 50 µm. (c) 30 µm.
![]() (a)
(a) ![]() (b)
(b) ![]() (c)
(c)
Figure 5. SEM of Cs2 at (a) 200 µm. (b) 50 µm. (c) 30 µm.
![]() (a)
(a) ![]() (b)
(b) ![]() (c)
(c)
Figure 6. SEM of Cs3 at (a) 200 µm. (b) 50 µm. (c) 30 µm.
![]() (a)
(a) ![]() (b)
(b) ![]() (c)
(c)
Figure 7. SEM of Cs4 at (a) 200 µm. (b) 50 µm. (c) 30 µm.
methods (1, 2, 3 and 4) at (200, 50, 30 µm) respectively. The Figs. show different surface morphology of each prepared chitosan according to the different method of preparation.
Figure 4 shows the surface morphology of Cs1 which presented smooth surface with small numbers of rocks structure on it, also Figure 5 shows the surface morphology of Cs2 which presented smooth and clear surface with heterogeneous rocks structure on it. Figure 6 shows the surface morphology of Cs3 which presented smoother and clear surface with some rocks structure on it and finally, Figure 7 shows the surface morphology of Cs4 which presented smoother and clear surface with small numbers of rocks structure on it.
4. Conclusions
Chitosan’s different degree of deacetylation can be obtained from deacetylation of chitin in strong sodium hydroxide solution at different period of time after extraction from shrimp shells, which waste shrimp shells in Egypt and this study is a preliminary study to evaluate various levels of deacetylated chitin for various applications. Chitosan (Cs2) possesses the highest thermal stability, crystallinity and degree of deacetylation which attributed to the increase of the sodium hydroxide concentration (70%), and its morphology shows crystals on its smooth surface.