Effect of Inorganic Salts on the Thermotolerance and Probiotic Properties of Lactobacilli Isolated from Curdled Milk Traditionally Produced in Mezam Division, Cameroon ()
1. Introduction
Probiotics are living microorganisms that play a beneficial role in human and animal health [1] . Regular intake or frequent inclusion of probiotic foods in the daily diet is increasingly encouraged in recent years [2] . Numerous research studies have demonstrated the beneficial effect of probiotics on the recovery of intestinal health, including the suppression of pathogens such as Salmonella enterica serovar Typhimuriun [3] [4] [5] [6] , Salmonella enteric serovar Enteridis, Campylobacter [7] [8] [9] Staphylococcus aureus [10] [11] [12] [13] and even Helicobacter pylori [14] [15] [16] [17] [18] . The administration of probiotics through food is one of the easy methods for taking probiotics for therapeutic purposes, the consumption of probiotic foods is recommended.
Most probiotic foods are dairy products such as yogurts, fermented milks in multiple forms, cheeses, but also fermented beverages and more rarely meat products [1] [19] [20] [21] [22] .
Artisanal and especially industrial production of probiotic foods generally involve processes taking place at high temperatures, reducing the survival and viability of the microbial strains involved and thus rendering the probiotic food ineffective when consumed as curative or preventive [23] [24] . The viability of the probiotic strains contained in these foods is an essential criterion for the effectiveness of these functional foods. Probiotic products should contain a fairly large number of viable cells in order to be effective after consumption [25] - [30] .
Very few studies have been conducted on the thermotolerance of probiotic strains. Some research carried out by Yang, Huang [31] have shown that Magnesium can improve the thermal tolerance of probiotic strains used in the food industry. Since these mineral elements are components of certain foods and play a very important role in the proper functioning of cells and in particular of probiotic microbes, it is necessary to test the effect of mineral elements such as Iron, Magnesium, Calcium, Manganese, Sodium, on the survival and viability of probiotic strains exposed to heat treatments in industrial processes for the production of probiotic foods and products.
The purpose of this research was to evaluate the protective effect of metal salts on the viability of probiotic strains exposed to processes involving high tempe- ratures.
2. Materials and Methods
2.1. Samples of Curded Milk Collection
Samples of the curds were purchased from M’Bororo peasants in Santa, Mezam Division of Cameroon. Curd is traditionally obtained after heating and natural fermentation of raw cow’s milk. A total of 20 samples of curdled milk were collected at a rate of 10 ml per sample and introduced into sterile bottles. The samples were then placed in a container containing pieces of ice to make the temperature low enough during transport. The samples thus obtained were transported to the Laboratory for the isolation of lactic acid bacteria with probiotic properties.
2.2. Isolation and Phenotypic Characterization of Potential Probiotic Bacteria from Traditionally Fermented Milk
Lactic acid bacteria (LAB) were isolated from curded milk by pour plate method on de Man Rogosa and Shape (MRS) agar (Oxoid, UK). For this purpose, ten- fold serial dilution of the milk samples was performed. The catalase negative colonies were selected and further characterized by Gram test and the determination of their carbohydrate fermentation profile using API 50 CHL Kit (BioMerieux, France). The Software APILAB plus version 5.0 was used for the identification of the catalase negative colonies.
2.3. In-vitro and In-vivo Study of Probiotic Properties
The ability to suppress the growth of pathogenic microorganisms and to resist acid and bile salts were the probiotic properties studied. The in-vitro ability to inhibit pathogenic bacteria was carried out by well diffusion method on Mueller Hinton agar. Salmonella enterica serotype Enteridis and Esherichia coli were used as indicator microorganisms. Selected lactic acid bacteria were cultured at 37˚C for 24 h in MRS broth (Oxoid, UK) supplemented with various concentration of mineral salts (0, 50, 100 mmol/L). The mineral salt used were: MgSO4・7H2O, NaCl, ZnCl2, CaCl2・2H2O (Sigma, UK). Fermented broth obtained were then centrifuged at 8000 g and kept at 4˚C. For the antimicrobial activity testing, Muller Hinton agar (Oxoid, UK) was poured in petri dishes, after solidification, 0.5 ml of the indicator microorganism was spread on the surface of each agar plate. After 15 min, wells of 5 mm diameter were aseptically made on the surface of Muller Hinton agar and 50 µl of the cell free supernatant (CFS) were inoculated into the wells.
Acid and bile-resistance were performed as we previously described [32] . Various concentrations of organic salts were added to assess their effect on resistance to acid and to oxgall-bile.
To test the probiotic properties of the selected lactic acid bacteria in-vivo, one day-old uninfected chicks were used, 10 of these chicks were infected with Salmonella enterica sp. This infection was carried out by oral gavage with 1.0 × 105 CFU/mL of Salmonella sp suspended in sterile water. Following this infection, the selected probiotic strains were administered by gavage with 1.0 × 109 CFU/ ml of each probiotic suspended in PBS buffer. After 30 days post-infection, the animals were sacrificed and microbiological analyzes were performed in the different organs of the gastrointestinal tract. Salmonellae and lactobacilli were counted in the colon using the Salmonella-Shigella (SS) and MRS agar respectively.
2.4. Thermal Treatment of Probiotic Strains
The probiotic strains to be studied were seeded in the MRS broth and incubated at 37˚C for 24 h. This culture was subsequently washed with buffered peptone water and re-suspended in 2 ml of 10% (v/v) Lactose containing 0, 20, 40, 50, 60, 100, and 500 mmol/L of MgSO4・7H2O, NaCl, ZnCl2, CaCl2・2H2O. The suspensions with the different concentrations of metal salts were homogenized using a vortex at ambient temperature (below 30˚C).
The whole was heated at 80˚C for 1 min. The number of viable and culturable microorganisms of the Lactobacillus strains was determined by counting on MRS agar and expressed as CFU/ml. The count was done for the strains that had undergone heating and those that did not. The survival rate was expressed as logN/N0, where N is the heat-treated population and N0 the population before heat treatment [31] .
2.5. Evaluation of Re-Growth of Lactobacillus Strains after Heat Treatment
In this experiment to evaluate the microbial growth of pre-heated cells, strains of Lactobacillus were cultured in MRS broth contained in test tubes and incubated at 37˚C for 24 h. Then centrifuged at 8000 g, 4˚C for 10 min. The pellet was collected and washed three times with buffered peptone water. The thus washed pellet was re-suspended in 2 ml of a 10% (w/v) lactose solution. The heat treatment was carried out by heating at 80˚C. for 1 min. The thus-heated microbial suspension was seeded into the MRS broth containing the various organic salts at various concentrations. The re-growth of the thus preheated strains was evaluated by measuring the change in optical density at 600 nm of the culture at 37˚C for 48 h. This spectrophotometric measurement was carried out using a microplate reader. The Baranyi model was used to fit the growth curves [33] . Two important parameters, the maximum specific growth rate 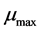 and duration of the lag phase
and duration of the lag phase 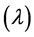 were calculated to estimate the re-growth of probiotic strains. The model was based in the assumption that the specific growth rate is practically constant for a phase. The difference between the growth rate and lag phase were studied by comparing their models obtained in the absence and in the presence of mineral salts.
were calculated to estimate the re-growth of probiotic strains. The model was based in the assumption that the specific growth rate is practically constant for a phase. The difference between the growth rate and lag phase were studied by comparing their models obtained in the absence and in the presence of mineral salts.
The Baranyi model used was based on the following differential equation [33] [34]
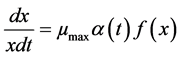 (1)
(1)
where 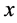 is the cell density and
is the cell density and 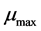 is the maximum specific growth rate (h−1);
is the maximum specific growth rate (h−1); 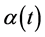 is an adjustment function describing the adaptation of the bacterial population to its new environment and
is an adjustment function describing the adaptation of the bacterial population to its new environment and 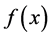 is an inhibition function describing the end-of-growth inhibition.
is an inhibition function describing the end-of-growth inhibition.
The solution to this differential equation is o
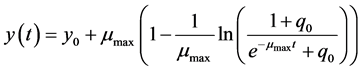 (2)
(2)
where 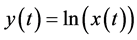 and
and 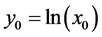
From this equation the lag time 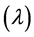 is given by:
is given by:
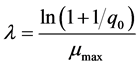 (3)
(3)
where 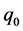 represents the physiological state of the inoculum. The plot of the Equation (2) was used for the determination of the maximum specific growth rate
represents the physiological state of the inoculum. The plot of the Equation (2) was used for the determination of the maximum specific growth rate 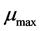 and duration of the lag phase
and duration of the lag phase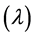 .
.
2.6. Statistical Analyses
The re-growth of probiotic strains in the absence and the presence of mineral salts were compared by ANOVA of the growth kinetic parameters. This analysis included the Fisher’s F-test and its associated probability p (F). All these statistical analyses were carried out using a computer’s program Statgraphic plus 5.0.
3. Results
3.1. Identification and Selection of LAB with Probiotic Potential
A total of 25 catalase negative isolates were isolated from the curded milk. A code was attributed to each isolate. Owing the fact that probiotic property most sought in our study was the in-vitro and in-vivo suppression of some food pathogens. Only two isolates codified LS3 and LM4 with these characteristics were retained for further studies. They were identified using API 50 CHL BioMerieux kit as strain of Lactobacillus casei and Lactobacillus plantarum. These two strains were selected based on their great capacity to significantly suppress two important food borne pathogenic bacteria, Esherichia coli and Salomonellaentericaserovar Enteridis (Figure 1) common in the several regions of Cameroon.
The in-vivo probiotic potential of the selected strains was demonstrated by administration of the isolate to one-day old non-infected chick model. The ceaca swabs were analyzed microbiologically by Salmonella count. The results are shown in Figure 2. A significant decrease (P < 0.05) in Salmonella was observed in the samples from chicks that have received L. casei (LS3) and L. plantarum
![]()
Figure 1. Inhibitory activity of L. casei (LS3) and L. plantarum (LM4) against E. coli and S. enterica sevovar Enteridis. Values are an average of three replicates ± standard deviation.
![]()
Figure 2. Salmonella count in caeca swabs from chicks pre-dosed with L. casei (LS3) and L. plantarum (LM4). The control were the chicks that did not receive administration of probiotic. Values are an average of three replicates ± standard deviation.
(LM4). These results suggest that L. casei (LS3) and L. plantarum (LM4) have contributed to a significant reduction of Salmonella in the gastrointestinal tract of the chicks post-inoculation.
3.2. Effect of Heat Treatment on the Viability of Selected LAB
The effect of heating on the survival of the selected isolates is shown in Figure 3. In general, the presence of minerals, Na, Mg, Ca and Zn increased the heat re- sistance of probiotic strains during heating. The increase of this heat-resistance was extended to varying degrees. There was a significant increase (P < 0.05) in
![]()
Figure 3. Survival of Lactobacillus casei (LS3) and Lactobacillus plantarum (LM4) in lactose solution supplemented with different concentration of NaCl, MgSO4, 7H2O, CaCl2・2H2O, ZnCl2 after heat treatment. Values are average of three replicates ± standard deviation.
the heat tolerance of probiotic strains (L. casei LS3 and L. plantarum LM4). The comparison of the four mineral salts used also showed that the effects of calcium and magnesium were the most important.
3.3. Effect of Mineral Salts on Bile Resistance of Selected LAB
The presence of mineral salts, in particular the calcium and magnesium salts, improved resistance to bile salts. In the presence of oxgall-bile and calcium, the survival of the strain L. casei (LS3) is greater than 95% after 4 hours of incubation (Figure 4). This result was similar with the L. plantarum (LM4) strain.
3.4. Re-Growth of Pre-Heated Microbial Strains
The ability to re-grow after heating was evaluated by measuring the growth kinetic parameters. Table 1 and Table 2 summarize the values of kinetic parameters λ and ![]() obtained from the Baranyi model. After heating the different strains of probiotic, their ability to regrow was evaluated by measuring the lag
obtained from the Baranyi model. After heating the different strains of probiotic, their ability to regrow was evaluated by measuring the lag
![]()
Figure 4. Effect of mineral salts on the bile-resistance of the strain Lactobacillus casei (LS3). Values are an average of three replicates ± standard deviation.
![]()
Table 1. Effect of the mineral salts on the re-growth of pre-heated probiotic Lactobacillus casei (LS3) strain.
Values are an average of three replicates ± standard deviation. Different superscripts letters for the same kinetic parameter indicate a significant difference between the values (analysis of variance test, P < 0.01).
![]()
Table 2. Effect of the mineral salts on the re-growth of pre-heated probiotic Lactobacillus plantarum (LM4) strain.
Values are an average of three replicates ± standard deviation. Different superscripts letters for the same kinetic parameter indicate a significant difference between the values (analysis of variance test, P < 0.01).
time and the specific growth rate. In the presence of the mineral salts, growth was improved, there was a significant reduction (P < 0.05) in lag phase duration (λ) and a significant increase (P < 0.05) in the specific maximum growth rate![]() . These results show that the protective effect of calcium was greater in L. casei (LS3).
. These results show that the protective effect of calcium was greater in L. casei (LS3).
4. Discussion
The isolation of many catalase-negative milk bacteria testifies the fact that milk is a favorable medium for the growth of lactic acid bacteria. The mesophilic lactobacilli were the dominant microflora of our samples of curded milk. These results are similar to those already described by Foschino, Invernizzi [35] . The selected strains (LS3 and LM4) meet the criteria required for the probiotics sought in our studies, in particular the antagonistic activity toward food borne pathogenic bacteria. Their ability to reduce (in-vivo) the proliferation of Salmonella entericaserovar Enteridis is of particular interest since this pathogen is commonly encountered in poultry and poultry products. In their study showing the importance of strains of probiotics in poultry La Ragione, Narbad [36] obtained similar results. These authors have demonstrated that L. Johnsonii (F19785) colonized the gastrointestinal tract of poultry which result in reduction of S. Enteridis and significant reduction of Clostridium perfringens also common in poultry. Vila, Fontgibell [37] in the same way demonstrated the reduction of Salmonella enteric serovar Enteridis by Bacillus cereus serovar Toyoi, These examples highlight the importance of probiotic in breeding, probiotic are actually recommended in breeding of different animals.
Our results show that the heat treatments which consisted in heating the probiotic strains in the presence of the lactose lead to a significant reduction in the viability of the strains. Loss of the viability of probiotic strains is a major problem encountered in the processing of probiotic foods and probiotic products. This loss of viability leads to a loss of the probiotic properties sought by manufacturers and consumers. Studies conducted by Coeuret, Gueguen [20] showed that most of the dairy probiotic products under the market have lost the viability of functional strains that qualified as probiotic foods. Most of these processes involve high temperatures. Hence the importance of thermotolerance of strains used in industrial processes.
An important research focus on probiotics currently is to find ways to maintain their stability and viability, or to increase their thermotolerance [31] . This is why we tested the effect of mineral salts on strains of lactobacilli isolated from curded milk in the locality of Mezam Division, Cameroon.
Our results highlight the protective effect of minerals such as calcium and magnesium on probiotic strains. This protective effect is due to the fact that calcium and magnesium ions have the property of stabilizing certain structural proteins and even enzymatic ones. Their presence may prevent the rapid denaturation of membrane proteins. Our previous work on thermostable amylases [38] showed that calcium acts by stabilizing enzymatic proteins at high temperatures. This property could explain the high thermotolerance of microbial strains in the presence of calcium, magnesium and other minerals.
Our study also shows that mineral salts increase resistance to bile salts of probiotic strains. This resistance could be explained by the stabilization of protein structures in the presence of calcium or magnesium.
5. Conclusion
This study reveals that the curdled milk traditionally produced by M'bororo peasants in Cameroon is a potential source of thermotolerant lactic acid bacteria with probiotic properties. Calcium and magnesium salts appear in our study as two elements significantly increasing the thermotolerance of probiotic strains. This study demonstrates that an adequate intake of calcium and magnesium in probiotic foods would help to maintain the viability of probiotic strains during and after industrial probiotic food production processes.
Acknowledgements
The authors would like to acknowledge the Biotechnology Unit of University of Buea, Cameroon, for providing facilities for research.