Developmental Changes in the Morphology of Western North Pacific Bryde’s Whales (Balaenoptera edeni) ()
1. Introduction
The body proportions of animals differ according to their adaptation to the environment [1] . Data on the body proportions of Balaenoptera species have been used in various studies, including those on taxonomy and stock identification [2] - [14] . Because body proportions change with growth, it is important to clarify the characteristics before using body proportions as an analytical tool. Developmental changes in body proportions have been reported for some Balaenoptera species, such as blue (Balaenoptera musculus), fin (B. physalus), and sei (B. borealis) whales [3] [15] [16] [17] . These studies have shown species-specific growth patterns, such as an increase in the length of the head region associated with a decrease in the length of the tail region in blue and fin whales [15] but no obvious trend in sei whales [16] . The prey species of Balaenoptera species range from planktonic crustaceans (copepods and krill) to schooling fishes, depending on the species [18] [19] [20] [21] . They feed using a unique system called “lung feeding” in which they engulf a large mass of water with prey and extrude only the water by filtering the prey items using their baleen plates [22] . The high energy cost of “lung feeding” and the necessity to create sufficient swimming speed leads to an optimum body proportion based on the feeding habits and body size (growth stage) of each individual [23] [24] [25] .
Bryde’s whale (B. edeni) is a medium-sized Balaenoptera species that inhabits tropical to temperate waters of both hemispheres at water temperatures > 20˚C [26] . Two stocks of Bryde’s whales are distributed in the western North Pacific Ocean: one in the East China Sea and coastal area of southern Japan and another in the off Pacific coast of Japan [26] . The offshore stocks of Bryde’s whales were regarded as a “southern form of sei whales” until the 1950s, and information on their body proportions is limited [27] [28] . Omura et al. [27] reported no significant difference in the body proportions between body length classes; however, this was because they measured a limited number of large individuals.
This study aimed to investigate developmental changes in the body proportions and sex-based differences in the growth pattern of western North Pacific Bryde’s whales using more than 700 specimens covering a wide body length range.
2. Materials and Methods
2.1. Materials and Measurements
In total, 726 Bryde’s whales collected by the Japanese Whale Research Program under Special Permit in the Western North Pacific-Phase II (JARPNII) conducted from 2000 to 2016 in accordance with Article VIII of the International Convention for the Regulation of Whaling were used in this study (Figure 1). Body parts were measured on board the research base vessel Nisshin maru. Body length (BL) was measured from the tip of the snout to the notch in the flukes in a straight line using a steel measuring tape to the nearest 1cm. In addition, 18 points were measured on different body parts, nine of which were measured along the body axis and nine were measured from point to point, based on the study by Mackintosh and Wheeler [15] (Figure 2). Measurements were made using steel measuring tapes or calipers according to the shape of the measurement point to the nearest 1 cm and of the dorsal fin length at base (DFLB), DFH, FW, SKL, and SKW to the nearest 0.1 cm.
![]()
Figure 1. Sighting locations (open circles) of Bryde’s whales collected during the 2000-2016 JARPNII surveys.
![]()
Figure 2. Measurement points for the body proportions of Bryde’s whales. Measurement points were selected based on the study by Mackintosh and Wheeler [15] .
2.2. Developmental Growth Analysis
Developmental changes in the growth pattern were examined by calculating the proportion of each measurement to the body length. The relationship of the proportion of each measurement to the body length was plotted, and a LOWESS curve was applied using R version 3.3.2 [29] to clarify the changes in accordance with growth. The relative growth pattern of each measurement was examined using the following allometric equation:
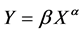
where X is the body length (cm), Y is the length of each measurement (cm), and α and β are the allometric coefficient and constant, respectively. Analyses were conducted for both sexes. Each measurement was classified into three growth patterns based on the value of the allometric coefficient: “positive” or “negative” when the allometric coefficient was significantly larger or smaller than 1 and “isometric” when the allometric coefficient was not significantly different from 1. Individual allometric equations were converted to a logarithmic form to examine the sex-based differences in the growth pattern. The α and lnβ of each measurement were analyzed using ANCOVA with sex as a factor and body length as a covariate. P-values of < 0.05 were considered statistically significant.
3. Results
3.1. Developmental Changes in Morphology
The proportion of the length from the tip of the snout to the blow hole (BH) was approximately 15% for a body length of 8 m. It increased to approximately 17% for a body length of 11 m in males and 12 m in females and subsequently stabilized (Figure 3). Measurements related to the head region (Eye, Ear, SKL, and SKW) showed similar trends; they increased to a particular body length and then stabilized. The length from the tip of the snout to the flippers (FL), which included the head and chest region, also increased to the same body length as the head region but subsequently decreased. The length of the chest region, calculated by subtracting SKL from the length from the tip of snout to the flipper (FL), stabilized in males and slightly increased in females until reaching the body length of around 11 m and subsequently decreased (Figure 4). Lengths related to the tail region (An, GA, and DF) decreased consistently by approximately 2% (Figure 3). The length between the notch in the flukes to the umbilicus (Um) and the end of the grooves (EVG), which included the tail and latter part of the abdominal region, decreased consistently by approximately 2%, although the gradient became moderate in the larger part of the body. The length of the abdominal region, calculated by subtracting FL and An from BL was constant at approximately 32% until reaching the body length of around 11 m in males and of 12 m in females and then rapidly increased to approximately 34% (Figure 4).
Dorsal fin length at base (DFLB) consistently decreased by 1%, whereas DFH stabilized until reaching the body length of around 11 m and then decreased (Figure 3). Both the width (FLW) and the depth (FLD) of the fluke decreased
![]()
![]()
Figure 3. Changes in the proportion of each measurement to the body length in Bryde’s whales. Red lines are LOWESS curves.
![]()
Figure 4. Changes in the proportions of the chest (upper) and abdominal (lower) regions to the body length in Bryde’s whales. Red lines are LOWESS curves. Length of the chest region was calculated by subtracting SKL from FL, and the length of the abdominal region was calculated by subtracting FL and An from BL.
consistently, and the extent of the reduction was approximately 2% for FLW and 0.5% for FLD. The proportions of the flippers (FTA, FTP, and FW) stabilized or increased slightly until reaching the body length of around 12 m and subsequently decreased.
3.2. Relative Growth Pattern
The relative growth pattern was positive for five measurements from the head region (BH, Eye, Ear, SKL, and SKW), although that for FL, which included the head and chest region, was isometric (Table 1). All measurements in the lower part of the body (An, GA, Um, EVG, and DF) showed a negative growth pattern. The dorsal fin (DFLB and DFH), flukes (FLW and FLD), and flippers (FTA, FTP, and FW) showed a negative pattern, except for FTA in males, which showed isometric pattern (Table 1).
3.3. Sex-Based Differences in the Growth Pattern
Most measurements in males and females showed the same relative growth patterns (Table 1). Significant sex-based differences were revealed by the lnβ of all measurements. Only one point, which was the length behind the dorsal fin (DF), showed a significant sex-based difference, as revealed by α (Table 1).
3.4. General Growth Pattern of Bryde’s Whales
Figure 5 shows the general growth pattern of Bryde’s whales estimated from this study. The head and chest region increased to approximately a body length of 11 m in males and of 12 m in females; subsequently, the length of the head region stabilized and that of the chest region decreased until the whales reached physical maturity. The length of the abdominal region was constant until a body length of 11 m in males and of 12 m in females and then rapidly increased. Lengths related to the tail region decreased consistently throughout the whole body length. The lengths of the dorsal fin and flukes also decreased consistently, whereas that of the flipper was constant up to a certain body length and subsequently decreased. Both sexes showed similar growth patterns, but the allometric equation coefficients differed between them.
4. Discussion
The slope of the fitting curves changed in some measurements at a certain body length, but the length at which the slope changed was approximately 1 m larger in females than in males. The body length at sexual maturity of male and female western North Pacific Bryde’s whales was reported to be 11.0 - 11.4 m and 11.6 - 11.8 m, respectively [11] . The body length at physical maturity was also larger in females than in males (13.5 m versus 13.0 m) [11] . The observed body length at which the slope changed was close to the body length at sexual maturity in both sexes, which might indicate that sexual maturity affects the changes in body proportions.
Growth-related changes in body proportions have been investigated in other
![]()
Table 1. Measurement points, coefficients and sex-based differences in the allometric equations and relative growth patterns of the western North Pacific Bryde’s whales. The probability indicates the significance of deviation of each allometric coefficient from a value of 1 (*:p < 0.05, **: p < 0.01) The relative growth pattern was classified as “positive” or “negative” when the allometric coefficient was significantly larger or smaller than 1 and as “isometric” when the allometric coefficient was not significantly different from 1.
![]()
Figure 5. Schematic diagram of growth dependent changes in the body proportions of a Bryde’s whale.
Balaenoptera species [3] [15] [16] [17] [23] [30] . Nakamura and Kato [30] examined the growth pattern of the skull of four Balaenoptera species using an allometric equation and reported positive growth in blue and fin whales, isometric growth in sei whales, and negative growth in western North Pacific common minke whales. Although the relative growth pattern of the head region of the Bryde’s whale was positive in this study, the increasing trend ceased at a certain body length. Blue and fin whales mostly eat krill, whereas sei whales feed on planktonic crustaceans (copepods and krill) and small fishes [18] [19] [20] . By contrast, common minke whales prefer to feed on schooling fishes [31] [32] . Bryde’s whales in the western North Pacific feed on krill and small schooling fishes [21] [32] . This divergence in feeding habits might have caused the variability in the growth patterns among Balaenoptera species.
The proportion of the abdominal region was constant until a certain body length and subsequently increased. Body length increases due to the elongation of the skull and vertebra. The growth of the vertebra along the body axis occurs at the cartilage between the centrum and epiphyses, and the growth ceases when the cartilage completely ossifies and the epiphyses fuses to the centrum [33] [34] [35] . Fusion begins at the anterior and posterior ends of the vertebral column in Balaenoptera species, proceeds to the central vertebra, and is completed at the dorsal vertebra [33] [34] [35] . The observed growth pattern of body proportion matched well with that assumed from the ossification pattern of the vertebra.
The proportions of the dorsal fin, flukes, and flippers decreased with growth. These parts affect swimming performance, such as rapid directional changes and swimming speed [1] . As the diet of Bryde’s whales includes krill and small fishes with poor swimming ability, the proportions of these parts become smaller, which provides fast cruising ability [1] .
Most measurements showed the same growth pattern between the sexes, but allometric equation coefficients differed between the sexes. A change in the slope of the fitting curve was observed for most measurements around sexual maturation length. A difference in the length at sexual/physical maturity would have led to a difference in the allometric equation.
Significant developmental changes and sex-based differences were detected in the body proportion of western North Pacific Bryde’s whales, which indicates the necessity of considering sex and growth stage when using body proportion as an analytical tool.
Acknowledgements
We thank all researchers and crew members who participated in the JARPNII surveys. T. Tamura of the Institute of Cetacean Research kindly assisted in preparing this manuscript. We also express gratitude to S. Inoue and F. Fukuhara for their contribution in preparing the figures. The JARPNII program was conducted with permission from the Japanese Fisheries Agency, Government of Japan.