Evaluation of Aqueous Product from Hydrothermal Liquefaction of Cardboard as Bacterial Growth Medium: Co-Liquefaction of Cardboard and Bacteria for Higher Bio-Oil Production ()
1. Introduction
Hydrothermal liquefaction (HTL) process converts wet biomass under subcritical temperature (280˚C - 374˚C) and pressure (10 - 25 MPa) to aqueous, solid char and gaseous products wherein process conditions, catalyst and biomass feedstock dictate product composition, distribution and yield [1] [2] . We have reported sub-critical HTL of lignocellulosic biomass (LCB) using homogeneous acid (Ca and Ni nitrate) catalyst [3] [4] . In our investigations, solid char was washed with acetone to extract higher hydrocarbons termed as heavy bio-oil (HBO) while dichloromethane extraction of the biocrude was performed to yield light bio-oil (LBO) which consisted of low molecular weight (C4-C11) oxygenated hydrocarbons. Under conditions optimized for maximum total bio-oil production, the LBO yield from cardboard feedstock was invariably low at 3.5 wt%. Considering the energy and solvent requirements, extraction of the LBO from biocrude is not economically appealing. The biocrude, hereafter referred as the aqueous product (AP) typically has up to 40% or more organic carbon from the initial feedstock. We have reported total organic content (TOC) in the range of 15 - 20 g・L−1 for AP from various homogeneously catalyzed LCB [3] [4] . In a continuous pilot scale HTL reactor with capacity to produce 42.9 million gallon gasoline-equivalent bio-oil from woody biomass, an estimated $ 25 M・yr−1 (33% of total operating costs) is projected for AP remediation and disposal [5] . Therefore, clearly, AP carbon recovery is critical for environment and better economics of large scale HTL reactors. Recycling of AP from LCB such as barley straw, aspen wood and dessert shrub were shown to increase bio-oil yields in the first few rounds. Zhu et al. reported 4 wt% increase in the bio-oil yield after 3 cycles [6] . In extended 10 cycle experiments by Biller et al with AP from K2CO3 catalyzed HTL of distiller’s grain, bio-oil yield increased from initial 44 wt% to 60 wt% with the first recycle [7] . More studies are needed to understand the pH changes, metal accumulation and the impact on bio-oil quality prior to introducing AP recycling in a large scale continuous HTL reactor system. Even then only some portions of the AP can be recycled to maintain favorable biomass to solvent ratio [8] . Therefore, a continuous HTL reactor operation will likely face persistent challenge of wastewater disposal. Alternatives such as catalytic hydrothermal gasification [9] , photoreforming for H2 production [10] , anaerobic digestion for methane production [11] have been investigated to recover organic components of the AP. Another route which is microbe mediated capture of AP carbon and nutrients is more appealing because it will simultaneously reduce the organic load of the waste stream and generate microbial feedstock for additional bio-oil [12] [13] [14] . However, the AP consists of heterogeneous and complex carbon compounds which vary in concentrations with different lignocellulosic biomass feedstock. Some for e.g. furans and phenols are toxic to most bacteria. Nelson et al reported significant dilution of AP from HTL of microalgae Chlorella vulgaris was required to reduce concentrations of growth inhibitory organic acids, phenols and heterocyclic compounds and support microbial growth. Compared with algal feedstock, higher quantities of phenolics are expected in AP from HTL of LCB. Moreover, AP from homogeneously catalyzed HTL of a biomass will also have bio-incompatible load of catalyst increasing nutrient imbalance and toxicity. To summarize, AP from catalyzed or uncatalyzed HTL of LCB is inherently challenging media to cultivate microbes. To achieve robust microbe mediated capture and recycle of carbon in AP, metal tolerant microbes with ability to utilize wide and unusual carbon sources need to be explored. To the best of our knowledge, only one study has employed an extremophile algae (Galdieria sulphuraria) to capture nutrients in AP from algal HTL [15] . Here, we investigate metabolically and genetically diverse Enterobacter species to capture carbon in AP from HTL of cardboard [16] [17] . Recently, novel Enterobacter species utilize complex substrates such as the kraft lignin and lignin model compounds as sole sources of carbon were isolated from soil, paper and pulp waste and ancient bamboo slips [18] [19] [20] . We have isolated the lignin degrading Enterobacter sp. RC202 (hereafter referred as RC202) from soil beneath decomposing wood in 4850 ft deep subsurface of the former goldmine in South Dakota [21] . The mine soil has high metal concentrations especially iron and low (<5 mg・kg−1) of organic carbon [22] . The RC202 has previously been shown to completely mineralize photocatalysed kraft lignin [23] . Therefore, we investigated it for culturability in AP from Ca(NO3)2 catalyzed HTL of cardboard to generate microbial biomass. Detailed analysis of the macro and micro nutrient composition of the AP was performed. Activated charcoal which has been used to detoxify hydrothermal hydrolysates of cellulosic biomass and starch was used to remove inhibitory compounds in the AP [24] [25] . We further demonstrate that co-liquefaction of microbial biomass with the cardboard feedstock induced a synergistic increase in bio-oil production and quality.
2. Materials and Methods
2.1. Materials
Corrugated cardboard from McMaster-Carr was mechanically shredded into pieces (80.75 ± 20.1 × 91.06 ± 17.5 × 2.16 ± 0.11 mm). Nitrogen (high-purity, HP grade) and helium (ultra-high-purity, UHP grade) were purchased from Linweld Inc., Rapid City, SD. All other chemicals used in this study were of analytical grade. Luria Bertini (LB) Miller broth was purchased from BioExpress Corporation, UT, USA. Glycerol stocks of Enterobacter sp. RC202 frozen at −80˚C were revived in LB following routine microbiology protocol. Activated charcoal powder USP grade (CAS 7440-44-0) was obtained from Spectrum Chemical Mfg. Corporation.
2.2. Hydrothermal Liquefaction of Cardboard and Bacteria for Bio-Oil
The HTL of 15 wt% corrugated cardboard was performed in a SS316 PARR reactor of 300 mL capacity at 250˚C for 60 minutes in the presence 5 wt% calcium nitrate (Ca(NO3)2∙4H2O) catalyst. The process for HTL is described elsewhere [3] [4] . Aqueous product (CbAP) was separated from the solid char by vacuum filtration using 589 black ribbon filter paper from Whatman Scleicher & Schuell. Filtrate from three batch experiments was pooled and centrifuged at 13,000 rpm, 18˚C for 10 min. The char free filtrate was stored at −80ºC until use. Hydrothermal co-liquefaction of the cardboard and the Enterobacter RC202 was performed in smaller 50 ml reactor designed using Swagelok port connectors. Figure 1 shows a schematic of Swagelok mini reactor. Mixtures of cardboard and bacteria in various ratios were prepared taking into account their dry weights. The water content of the RC202 was experimentally determined to be 70 wt%. The total weight of the mixed feedstock was 1.0 gram. A slurry of cardboard pieces and bacterial cell pellet and 5 wt% (Ca (NO3)2∙4H2O) catalyst in 10 ml water was introduced in the reactor. The reactor was heated to 325˚C for 60 minutes using a sand bath. The AP and char were separated by the same filtration protocol described above for products from the PARR reactor. The LBO was extracted from AP using toluene and HBO was extracted from the char using acetone. The bio-oils were dried under stream of N2 for 24 hours and then weighed. The schematic in Figure 2 shows a flow diagram to integrate bacteria mediated AP carbon capture and recycle in a continuous HTL reactor system. It also provides a flow sheet of methods used to produce light and heavy bio-oils from cardboard and bacterial feedstock. The bio-oils (LBO and HBO) and char yields were calculated using following formulae:
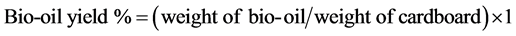 ……….. (1)
……….. (1)
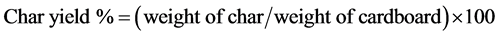 …………. (2)
…………. (2)
The elemental composition (C, H, N and S) of the bio-oil samples was performed by Atlantic Microlab, Inc. Ga. The weight percent oxygen was obtained by subtracting the C, H, N and S wt% from 100. The Boie formula given below
![]()
Figure 1. Swagelok mini-reactor system for hydrothermal co-liquefaction of microbial biomass and cardboard.
![]()
Figure 2. Flow diagram to integrate bacteria mediated AP carbon capture and recycle in a continuous HTL reactor system.
was used to calculate the higher heating values (HHV) of the bio-oil [26] .
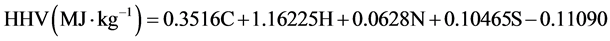 ….(3)
….(3)
2.3. Activated Charcoal (AC) Treatment of CdAP
The CbAP was mixed with various amounts of activated charcoal (10 - 30 mg・mL−1). The mixture was stirred at ambient temperature by magnetic bead at 100 rpm in the dark for 8, 12 and 24 hrs. AC particles were separated by centrifugation at 13,000 rpm for 30 min. The supernatant was filtered through a 589 black ribbon filter paper. The clear filtrate termed as the detoxified aqueous co-product (DT-CbAP) was stored at −80ºC until use.
2.4. Analysis of Chemical Composition and Nutrient Stoichiometry of CbAP
A laboratory TOC analyzer GE (Sievers InnovOx) equipped with non-dispersive CO2 detector measured the inorganic carbon (IC) from the total dissolved carbon. The reagent was 6M phosphoric acid (GE Sievers) whereas 30% w・v−1 sodium persulfate was used as the oxidizer. The difference between the total carbon and the IC provided the TOC. The initial carbon content of cardboard of 46.8 wt% is reported earlier [3] .
2.5. Macro and Micronutrient Analysis.
The CbAP samples were analyzed for nitrate/nitrites or ammonia and orthophosphates by water testing lab in University of Missouri, Columbia. The samples were acidified using 3 vol% concentrated nitric acid and analyzed for micronutrients by Agilent 7800 ICP-MS.
Media supernatants of abiotic control and RC202 inoculated DTP-CbAP were freeze dried to powder. The dry powders were suspended in equal volume of 95% vol・vol−1 aqueous acetone. The samples (1 ul) were loaded on GC-MS (Agilent 7890 GC; 5975C VL mass detector) equipped with HP-5 ms capillary column (5% phenyl, 95% dimethylpolysiloxane, 30 m × 0.25 mm × 0.53 mm). Helium was the carrier gas flown at a rate of 1 ml・min−1. The oven temperature was held at pre-injection 40˚C for 10 min, then increased at a rate of 2˚C・min−1 to 170˚C, held for 5 min followed by 6˚C・min−1 to 250˚C (10 min hold time) and finally increased by 15˚C・min−1 to 300˚C (10 min hold time). The MS scan range was m/z 35 - 550 at normal scan rate. Compounds were identified by matching m/z values with those in the National Institute of Standards and Technology (NIST) MS Library Search (2008, version 2.0 f). The C1-C3 carboxylic acids in DT-CbAP were quantified by Shimadzu HPLC equipped with an Aminex HPX-87H column (300 mm × 7.8 mm) and a SPD-10A (UV-VIS) detector set at 210 nm. The mobile phase was 0.005 M H2SO4 was run at flow rate of 0.5 ml・min−1. The column temperature was maintained at 60˚C and sample injection volume was 5 µl. A five point calibration plot for each acid was obtained using HPLC grade reagents.
2.6. Enterobacter sp. RC202 Culture in CbAP
The initial pH of DT-CbAP was 3.7. It was adjusted to 7.2 with 2 M NaOH. Dilutions (5% - 80% vol・vol−1) of AP were made in 10 mM potassium phosphate buffer pH. Precipitate formed was removed by centrifugation at 13,000 rpm for 20 min. The buffered media (DTP-CdAP) was supplemented with 50 mg・mL−1 sterile yeast extract just prior to adding bacterial inoculum. RC202 cells acclimatized and maintained in minimal media containing wt・vol−1 each of 0.05% kraft lignin, 0.5% glucose supplement and 0.1% yeast extract were washed in sterile saline and inoculated in the 50 ml each of CbAP, DT-CbAP and DTP-CbAP at a cell density of 2 × 107 cells ml−1. The cultures were incubated in dark to 0 hrs, 24 hrs and 48 hrs at 25ºC, in a shaker incubator adjusted to 150 rpm. The cells were separated by centrifugation at 4000 rpm for 20 min. Cell pellets were dried in an oven at 60ºC for 48 hrs and then weighed. Cell free growth media was analyzed by GC-MS for carbon substrate profile and utilization.
3. Results and Discussion
3.1. Detoxification of CbAP
The Enterobacter RC202 metabolizes complex carbon compounds formed from photocatalysed kraft lignin [23] . Even so, attempts to culture RC202 in “as-is” CbAP from catalyzed and non-catalyzed HTL reactions did not succeed. On the contrary, a steady decline in cell number was observed with increase in incubation time. Activated charcoal which has broad adsorption capability and has been previously used to detoxify lignocellulosic biomass hydrolysates was selected to remove inhibitory compounds [24] [25] . Minimal AC loading and short contact time were important factors considered to prevent excessive carbon loss in detoxification process. Amongst the 3 different loadings and contact times tested, CdAP treated with 10 - 30 mg・mL−1 of AC for 8 or 12 hrs retained light amber color while only the AP treated for 24 hrs with 30 mg・mL−1 had a “clean water” appearance. The CdAP treated with less than 30 mg・mL−1 did not support cell growth (ΔOD 600 < 0.05) in 48 hrs incubation at 25ºC and was not included in further studies.
3.2. Macronutrient and Micronutrient Composition of CbAP
The stoichiometry of carbon, nitrogen and phosphorus atoms (C:N:P ratio) and quantities of micronutrients in growth media affect bacterial growth and cell density. The macronutrient compositions (Table 1) of 80 vol% DTP-AP and Cd- AP were compared with literature values for LB broth which is routinely used to cultivate Enterobacter species.
The initial TOC of CdAP was 20,243 mg・L−1 which was reduced to 9176 mg・L−1 in the 80 vol% DTP-CdAP. Compared with most algae, LCB is inherently low in N and P. For example, the N content of pinewood and cardboard is 0.22 and 0.08 wt%, respectively. In contrast, the N content of algae Chlorella vulgaris is 6.2 - 7.7 wt% [27] . Therefore, the AP from HTL of algae has much higher N and P content compared with AP from LCB. The N content of CbAP from HTL without the catalyst is only 80 mg・L−1 (data not shown). However, in this study, HTL of cardboard was performed in the presence nitrate salt catalyst which increased the N content of CbAP to 981 mg・L−1 of which only 25 mg・L−1 was in form of more bio-available ammonium-N. The P level too was found to be low 8.35 mg・L−1. Diluting DT-CbAP in sodium phosphate buffer improved the orthophosphate to 21.2 mg・L−1. These modifications improved the C:N:P ratio of DT-CbAP from 7469:80:1 to 443:35:1 for the 80 vol% DTP-AP bringing it closer to the reported 189:49:1 ratio of LB. In higher dilutions the actual concentration of C, N and P decreased but the stoichiometry remained same. The C:N of 12.23 is much higher for DTP-AP compared with the LB which has C:N of 3.85 indicating that more carbon is available for nitrogen present in the DTP-AP. However unlike in the LB media, carbon substrates in LCB AP are much less bio-utilizable. The micronutrient composition shown in Table 2 indicates that with exception of Na, K and Cu the CbAP has adequate micronutrients to support bacterial growth. High calcium (Ca) levels of 3243 mg・L−1 from employing Ca(NO3)2 catalyst decreased by 22% to 2532 mg・L−1 after AC treatment and dilution in phosphate buffer. Even then, the Ca concentration is 255 fold higher than in LB media. The DTP-CbAP also contains higher levels of magnesium (Mg), potassium (K), manganese (Mn), iron (Fe), cobalt (Co) and chromium (Cr) compared with LB media. However, the RC202 was isolated from highly metalliferous mine soil, we have observed that it is more metal tolerant, com-
![]()
Table 1. C, N, P content of CbAP, DTP-CbAP and Luria broth.
pared with other Enterobacter species (unpublished data). Based on HPLC quantitation of 4 major C1-C3 acids, the carbon contribution was calculated (Table 3). The analysis revealed that the combined 2588 mg・L−1 of formic, acetic, lactic and propionic acids were present in the CdAP. Only 333 mg・L−1 remained in the DT-CdAP following detoxification indicating that more than 80% of the acids were adsorbed on activated charcoal.
GC-MS analysis of the abiotic and RC202 inoculated DTP-CbAP was conducted to identity carbon compound profile and those utilized by the RC202. Only those compounds with relative peak areas (RPA) > 0.5% of the total area are shown in Table 4. The sum of RPA of compounds is 90.21%. Data from 3 different batches of DTP-CbAP prepared from the same biomass feedstock and liquefied under identical reaction conditions showed significant variations in tentative identities of compounds with RPA range between 0.2% - 5%. The GC-MS analysis highlights the heterogeneous and the complex nature of carbon substrates in the CbAP. Some of these such as the heterocyclic amines (e.g. piperdine derivatives) were not removed by activated charcoal treatment and are toxic to Gram-negative and Gram-positive bacteria.
Although activated charcoal treatment will be inexpensive and easy to integrate with continuous HTL operation, improved methods to specifically remove or reduce only the toxic or growth inhibitory components in AP are needed.
![]()
Table 2. Micronutrient composition of CbAP, DTP-CbAP and Luria broth.
![]()
Table 3. HPLC quantitation of the C1-C3 carboxylic acids in the CbAP and DT-CbAP.
![]()
Table 4. GC-MS analysis of abiotic and RC202 inoculated DT-CbAP growth media supernatant.
3.3. Growth of Enterobacter RC202 in CbAP
Detoxified CbAP diluted at 5, 15, 40, 60 and 80 vol% in phosphate buffer (DTP- CbAP N%) and undiluted CbAP from which LBO fraction was removed by dichloromethane extraction (CbAP-LBO) were inoculated with Enterobacter RC202. Figure 3(a) shows increase in the dry cell weight (Δ DCW) of RC202 after 48 hrs of incubation at 25˚C. The DCW of RC202 cultured in LB media under identical conditions increased 70 fold in 24 hrs. The data in Figure 4(a) shows that the DTP-CbAP-80% supported only 1.7 fold increase in DCW which is only slightly better than the 1.2 fold obtained with the highly diluted DT- CbAP-5%.
More concentrated DT-CbAP 40 vol% and 60 vol% showed reduced growth with only 5.3 and 3.2 fold increase in dry cell weight. The highest cell yield was
![]() (a)
(a)![]() (b)
(b)
Figure 3. Culturability of Enterobacter RC202 in DT-CbAP-LBO and diluted (5 - 80 vol%) DT-CbAP in phosphate buffer and (b) Growth pattern of RC202 in CbAP-LBO and DTP-CbAP-15 vol%.
obtained with 15 vol% dilution of DTP-CbAP which supported a 9.4-fold increase in cell density. Growth pattern of RC202 cultured in the DT-CbAP-80% shows steady increase in cell density up to 24 hrs and not much increase thereafter. The undiluted CbAP without the LBO fraction supported meagre (0.6 fold) growth while no growth was observed in the CbAP with LBO. Furan derivatives constituted ~25% of the total peak areas in GC-MS analysis of the LBO from cardboard [3] . It appears that either separating the LBO fraction or diluting it is necessary to reduce the toxicity of AP. Even then considering its complex carbon substrate profile only a modest microbial biomass harvest should be expected from AP based growth media.
3.4. Co-Liquefaction of Bacteria with Cardboard Feedstock
We sought to understand the advantages of recycling microbial biomass with
cardboard feedstock for bio-oil production. Gai et al. have shown that co-lique- faction of rice husks with microalgae increased extraction of bio-oil from rice husks [28] . Results in Figure 4 demonstrate the synergistic effects of co-lique- faction of RC202 and cardboard feedstock mixed in different ratios. These experiments were repeated twice with errors within ±5%. The total bio-oil (LBO and HBO) from bacteria alone is 36.4% higher than from 100 wt% cardboard. Bio-oil yields increased with increase in bacterial biomass in the mixed feedstock. The maximum bio-oil yield increase of 33 % was obtained from 1:1 feedstock mix. A similar increase of 32 % was from the 1:3 bacteria: cardboard feed. These results imply that even small quantities of microbial biomass added to lignocellulosic feedstock can improve bio-oil production. Similar synergy in microalgae and high density polyethylene co-liquefaction was shown to improve bio-oil yield by 44.81% [29] [30] . Higher heating Values (HHVs) calculated on the basis of C, H, N, O and S content reflect the oil quality. The HHVs determined for various bio-oils produced are shown in Table 5. The bio-oil from cardboard is more
![]()
Figure 4. Bio-oil Yields from co-liquefaction of bacteria and cardboard. Elemental analysis of bacterial dry cell pellet revealed wt% C = 50 while that of cardboard is 48 wt%. The total organic carbon content of the singular and mixed feedstock was at ~33333 mg・L−1. Expected yield was calculated from bio-oil quantities produced by single feedstock and projected quantity when combined with the 2nd feedstock.
![]()
Table 5. Elemental analysis and higher heating value of bio-oils from co-liquefaction of bacteria and cardboard.
oxygenated as evident from its high 20.21 wt% oxygen, while that from bacteria has only 11.08%. Combining bacteria with cardboard feedstock in 1:1 ratio improved energy density of the bio-oil. It is seen that HHV of bio-oil from feedstock composed of with 1:1 ratio of bacteria to cardboard was 34.11 MJ・kg−1 which is higher than HHV of bio-oil from feedstock with B:C ratio of 1:3 and 0:1.
4. Conclusion
This study supplements current efforts to utilize carbon in AP from catalyzed HTL of LCB with specific example of cardboard and Ca(NO3)2 catalyst. The investigation primarily highlights the challenges in cultivating bacteria in the AP and the advantages in recycling bacterial biomass for higher bio-oil production and quality. Activated charcoal, N and P amendment as well as dilution were necessary to reduce inhibitory compounds and balance macronutrient stoichiometry. The highest bacterial cell yield was obtained from the activated charcoal treated 15 vol% DT-CbAP. Higher cell densities in more concentrated DT-CbAP may be achieved by either alternating aerobic and anaerobic culturing conditions or using co-cultures of Enterobacter species. Co-liquefaction of the bacteria and cardboard produced 33% increase in bio-oil with HHV of 34.73 MJ・kg−1. The synergistic interaction between the LCB and bacterial feedstock increases the possibility to include microbe mediated capture and recycle of AP carbon in a continuous reactor system for higher hydrocarbon efficiency and possibly low wastewater remediation requirement.
Acknowledgements
The authors gratefully acknowledge the financial sup-port from US AFCEC under the grant BEAR?EST#FA4819-14-C-0004.