Hydrogeochemistry of Groundwater from Different Aquifer in Dimbhe Command Area of Ghod River, Maharashtra India ()
1. Introduction
The biosphere’s main available freshwater and essential resource is groundwater. The world depends on groundwater for intake water supply, green growth, and industrialized purposes as well as for worldwide foodstuff safety. Nearly world’s thirty-three percent inhabitants depend on subsurface water for drinking purpose [1] . The geochemistry of water is depends on rock type of an area, also the amount of chemical weathering of rock and man induced factor like land-use affect the chemistry of groundwater [2] . The combination of productive soil, moderately undulating topography with the superficial water level and widespread of the aquifer with plentiful and easily accessible of water make the groundwater the main source of water among the residents in the Dimbhe command area of Ghod river basin. About 75% populations living in the Ghod river basin are dependent on surface and subsurface water for domestic, farming and industrial activities. The subsurface water requires negligible treatment and remains nearly unaffected throughout extended drought period. However, the basaltic aquifers are generally delicate, easily exhausted due to manmade actions and over use of groundwater [3] . With the growing populaces distributed over a large geographical area, the demands of groundwater increased in command area [3] [4] .
In industry and agriculture activities, there has been increasing concern about the quantity and quality of groundwater resources [5] . For sustainable management and safeguard of precious groundwater resources, the categorization and understanding of the environmental evolution of subsurface geochemistry are crucial to explain their element nature and its relation [6] . Presently, the hydrogeochemistry study of subsurface water in this area has not been examined in depth on a basin-wide scale and poorly unwritten. The aim of this paper is to portray the groundwater class in the Ghod river basin in multi-layered aquifer using geochemical analysis.
2. Material and Methodology
2.1. Study Area
The River Ghod originates in the Bhimashankar area at approx. 1090 m above sea level. It is a tributary of the River Bhima that flows in an east-southeast direction for approximately 200 km before its confluence with the River Bhima. It flows from the northern side of the Bhimashankar hills. The Ghod River itself has two tributaries-River Meena and River Kukadi. There is a long canal constructed along the Ghod river bank. The Dimbhe dam is located in the Ghod basin and is part of the Kukadi project. Figure 1 is location map of the study area. The right side of figure shows the India showing Maharashtra state having Pune district and study area.
The Dimbhe reservoir is designed to irrigate hectares of land and generate 5 MW power through a powerhouse built at the downstream of the dam. The study area experiences good to high rainfall during the southwest monsoon (June-August) and northeast monsoon (October-December) seasons. The mea- sured rainfall varies from 650 to 900 mm/day. The temperature of the area varies from 20˚C to 34˚C and the maximum in the summer seasons.
The study area is occupied by the horizontal flows of basalt, showing Ajanta, Karla, Lower Ratangarh and Upper Ratangarh Formation (Figure 1).
2.2. Sampling and Analytical Procedures
The total of 46 groundwater samples was collected from shallow (<10.0 m depth), and deep aquifer (>10.0 m depth). The systematic sampling method was
![]()
Figure 1. Location map of study area showing sampling location.
chosen in order to indicate the groundwater quality in the study region (Figure 1). The subsurface samples were collected in prewashed sampling bottle. The physicochemical parameter measured in the field immediately after were pH, temperature, electrical conductivity (EC), and total dissolved solids (TDS) using the handheld meter and data-logging. All samples brought to the laboratory and stored at 4˚C. The samples were collected in 1 Liter and 100 ml sampling bottles. The 100 ml sample is acidified with nitric acid (HNO3) to maintain a pH of less than 2 to reduce adsorption of metals to container walls and decreases biological activity. Future the samples were filtered through 0.45 μm membrane filter to remove unwanted suspended particle. All the groundwater collection method and the water sample analysis followed standard procedure [7] .
The Ca, Mg, HCO3 and Cl− were analysed by titrimetric methods [7] . The Na and K concentrations were determined by flame photometric method while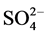 ,
, 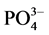 , and
, and 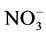 were analyzed by using UV-VIS spectrophotometer. The Fe and Mn usingwere determined by a nano-colorimeter (500D). All concentrations are expressed in milligrams per liter (mg/L), except for pH and EC. Analytical precision of the major ionic constituents was measured by the normalized inorganic charge balance. The charge balance errors in all groundwater samples are within ±5%, which is considered to be acceptable [8] . Aquachem software was used for the analysis.
were analyzed by using UV-VIS spectrophotometer. The Fe and Mn usingwere determined by a nano-colorimeter (500D). All concentrations are expressed in milligrams per liter (mg/L), except for pH and EC. Analytical precision of the major ionic constituents was measured by the normalized inorganic charge balance. The charge balance errors in all groundwater samples are within ±5%, which is considered to be acceptable [8] . Aquachem software was used for the analysis.
3. Result and Discussion
3.1. Hydrogeochemical Facies
Hydrogeochemical diagrams are aimed at facilitating interpretation of evolutionary trends, particularly in groundwater system, when they are interpreted in conjunction with distribution maps and hydrochemical sections [9] . The Piper diagram in Figure 2(a) shows the prominent groundwater facies of the shallow aquifer is Ca-HCO3 and Na-HCO3 indicating fresh and mix-water types respectively. In deep groundwater aquifer (Figure 2(b)), Na-HCO3 type water is dominant. Ca-HCO3 facies indicate that the groundwater samples are associated with calcite solution. The majority of groundwater resources were Ca + Mg − HCO3 + CO3 dominant as the dissolution of primary silicates due to process of chemical weathering [10] . Despite this, the water from Ca − Mg rich basaltic flows, the surface water is always deprived with Ca and Mg because of setting of those ions in their carbonates as the result of alkaline nature of water. Also, majority of the wells occupies the inner diamond area as rock dominance prevalence. Dug wells or shallow groundwater are evenly embedded in diamond area, while bore wells or deep groundwater bowing towards Ca + Mg − HCO3 + CO3 due to long residence time available for rock-water interactions [11] .
It is observed that changing of Ca + Mg − HCO3 + CO3 to Na + K − SO4 + Cl is more in bore well or deep aquifer than dug well or shallow aquifer [12] . This is due to greater depth of bore well wherein high residence time groundwater exists due to the increased rock-water interaction. However, surface water is having high evaporation that results into feeble displacement in Ca + Mg − HCO3 + CO3 to Na + K − SO4 + Cl water facies due to less rock-water interaction [12] .
3.2. Sodium Adsorption Ratio (SAR)
The groundwater quality from shallow and deep aquifer were classified and compared with sodium absorption ratio (SAR). The SAR calculate the ions of sodium (Na+) to calcium (Ca2+) and magnesium (Mg2+) ratio in groundwater samples. Sodium hazard of irrigation water is important in classifying the water for agriculture purposes because sodium concentration can reduce the soil permeability and soil structure [13] and calculated by using Equation (1) [14]
 (1)
(1)
The USSL graphical diagrams of irrigation water plotted the SAR and electrical conductivity (Figure 3). Based on USSL diagram [15] , groundwater samples from shallow aquifer fall in the C1-S1 (low salinity with lowsodium), C2-S1 (medium salinity with low sodium) and C3-S1 (high salinity with sodium). Hence, the groundwater from shallow aquifer can be used on most crops for irrigation purposes. For the deep aquifer, samples fall in the medium salinity and low alkalinity region C2-S1. Only one of the samples falls in the region C3-S1, which indicates high salinity with low alkalinity. Generally, the study area indi-
![]()
Figure 2. Piper diagram in (a) shallow groundwater,(b) deep groundwater.
![]()
Figure 3. USSL graphical diagrams of shallow groundwater and deep groundwater.
cates low to high salinity and low to medium alkalinity water, which can be used for irrigation in almost all types of soils with a little danger of exchangeable sodium.
4. Conclusion
Results of the analysis show that the shallow groundwater type is dominated by Ca-HCO3 and Na-HCO3 and deep groundwater Na-HCO3 as basaltic groundwater. The Na-HCO3 facies could be due to albite solution of weathered Gyroliteand Siderophyllite of the study area. The bicarbonates are mainly derived from carbonate mineral and silicate weathering. However, salinity in study area is to be added with increase in annual rainfall which offers sufficient aqueous medium for rock/soil-water interaction under in semi-arid climatic regime. The process of rock dominance is dominant followed by precipitation and evaporation. Finally, the groundwater of the Ghod river Basin is suitable for drinking, domestic and agriculture irrigation use. The people from study area will have adopted sustainable water use plan for surface and subsurface water to monitor and control groundwater quality for anodyne usage of the resource.
Acknowledgements
The authors are thankful to Principal, N. Wadia College Pune, India for extending help to use the department laboratory for computing facilities.