Catalyst Free One-Pot Synthesis of Chromeno Quinolines and Their Antibacterial Activity ()
1. Introduction
Multi-component reactions (MCRs) have a great role in the organic synthesis. These are one step reactions, where the reactants are subjected into a single reactor, to form a desired product with high yields, without any intermediate formation. Its importance lies mainly in the synthesis of medicinally potent compounds and its convenient preparation than the conventional methods to form privileged scaffolds in a single step process, thereby having great advantage over convergent and conventional synthesis [1] [2] [3] .
The product molecules consisting of quinoline and chromene moiety have a broad range of application with biological activity such as anti-malarial, anti-asthmatic, anti-inflammatory and anti-bacterial property [4] [5] . Chromene is the privileged structural component for various natural products consisting of photochemical properties. It is the backbone of many polyphenols found mostly in alkaloids, flavanoids, tocopherols and anthocyanins [6] . The chromene derivatives are potential anticancer agents [7] .
From the literature survey, taking xanthenes as a reference some of methods have been developed for the synthesis of dimethyl-dihydro-7H-chromeno[3, 2-h]quinolin-8(9H)-one derivatives. Xanthenes are usually prepared using 2- naphthol, aldehydes and dimidone as reactants in presence of various catalysts such as Bronsted acidic ionic liquid Triethylamine-bonded sulfonic acid [8] , Ionic Liquid Pyrazinium Di (hydrogen sulfate) [9] , ionic liquid 1, 3-disulfonicacid imidazolium hydrogensulfate [10] , N, N’-dibromo-N, N’-1, 2-ethane diylbis (p- toluenesulfonamide) [11] , 3-sulfobutyl-1-(3-propyltriethoxysilane) imidazolium hydrogen sulfate on silica-coated Fe3O4 nanoparticles [12] ammonium chloride [13] , silica-bonded imidazolium-sulfonic acid chloride [14] , ionic liquid sulfonic acid functionalized pyridinium chloride [15] , ZnO nanoparticles [16] , ceric ammonium nitrate [17] under solvent free conditions and Bismuth (III) nitrate using water as solvent [18] , phosphomolybdic acid using dichloroethane as solvent [19] , ceric ammonium nitrate using DCM-ethanol as solvent [20] , Thiamine hydrochloride using hexadecyltrimethylammonium bromide (CTAB) in aqueous micellar form as solvent [21] as catalysts.
Though these methods involve their own limitations like longer reaction times [8] [11] [12] [17] using toxic reagents [8] [9] [10] [11] [14] [19] and [21] , difficulty to separate the catalyst [13] and [18] and catalyst degradation during the process of the reaction which cannot be recovered [17] [19] and [20] . The main objective of our research is the organic synthesis, which involves green procedures, short reaction time, low temperature conditions, higher yields, and economically desirable processes without any use of catalyst.
In continuation to the synthesis, characterization and catalytic application of nano copper and cobalt ferrite catalysts was reported by us in the preparation of 2, 4, 5,-trisubstitued imidazoles by one-pot synthesis [22] , tri and tetra substituted imidazoles under ultrasonication catalyzed by nano copper ferrite [23] , microwave assisted synthesis of β-acetamido ketones, catalyzed by nickel cobalt ferrite [24] poly substituted pyridine derivatives with copper ferrite [25] 4H- Pyrano[3, 2-h]quinoline derivatives under microwave irradiation with nano cobalt ferrite [26] . New greener reactions paths have been investigated.
Now we report an efficient greener synthesis of dimethyl-dihydro-7H-chro- meno [3, 2-h]quinolin-8(9H)-one derivatives through cyclization of aromatic aldehyde, dimidone and 8-hydroxy quinoline (Scheme 1) through one pot condensation method.
2. Experimental
2.1. Chemicals and Apparatus
All chemicals used in this process are of AR grade fine chemicals, without any further purification. The synthesized dimethyl-dihydro-7H-chromeno [3, 2-h]
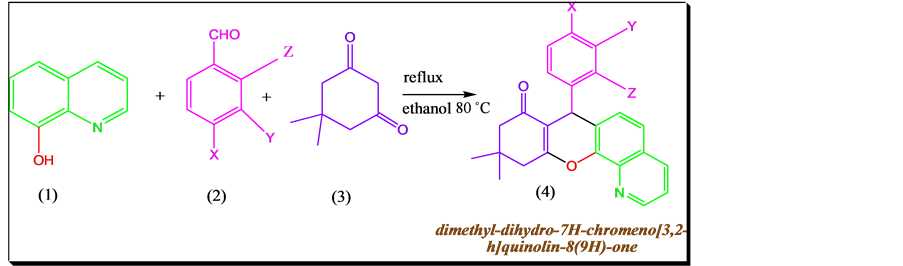
(a) X = Cl, Y = H, Z = H; (b) X = OH, Y = OC2H5, Z = H; (c) X = OH, Y = H Z = H; (d) X = CH3, Y = H, Z = H;(e) X = H, Y = H, Z = H; (f) X = NO2, Y = H, Z = H; (g) X = H, Y = H, Z = NO2; (h) X = H, Y = H, Z = OCH3;
Scheme 1. Synthesis of dimethyl-dihydro-7H-chromeno[3, 2-h]quinolin-8(9H)-one derivatives.
quinolin-8(9H)-one derivatives were characterized by FTIR, MASS and 1H NMR. FTIR spectra recorded on a (Perkin Elmer Spectra-880) spectrophotometer by using KBr pellets in the region 400 - 4500 cm−1 and 1H NMR spectra was characterized by 400 MHz-(Bruker Avance) in CDCl3 solvent and MASS spectra was recorded at 70 eV (MASPEC low resolution mass spectrometer).
2.2. General Procedure for the Synthesis of Dimethyl-Dihydro-7H-Chromeno [3, 2-h]Quinolin-8(9H)-One Derivatives
The one pot synthesis of dimethyl-dihydro-7H-chromeno [3, 2-h]quinolin- 8(9H)-one derivatives was carried out in 250 mL round bottomed flask by taking Equimolar quantities of aromatic aldehydes (10 mmol), dimidone (10 mmol) and 8-hydoxyquinoline (10 mmol) and 15 mL of ethanol were mixed together and the flask was placed in oil bath over a hotplate consisting of magnetic stirrer and kept for reflux at 80˚C. The progress of the reaction was monitored by TLC using mobile phase (n-Hexane: ethyl acetate 3:1). The formed product mixture was cooled to room temperature and ethyl alcohol added until the product was dissolved. The products were recrystalized with ethanol and characterized and compared by FT-IR, 1H NMR and MASS spectral techniques are tabulated in Table 1.
3. Results and Discussion
The procedure involves the cyclization of aromatic aldehyde, dimidone and 8-hydoxyquinoline is described as model reaction shown in Scheme 1. The attainability of formation of chromeno [3, 2-h]quinolin-8(9H)-one derivatives and the reaction conditions are tabulated in Table 2.
3.1. Comparative Study for the Synthesis of Dimethyl-Dihydro-7H-Chromeno [3, 2-h]Quinolin-8(9H)-One Derivatives with Other Catalysts
Reaction times for the formation of chromeno[3, 2-h]quinolin-8(9H)-one derivatives with various catalysts are presented in Table 3. It is observed that with
![]()
![]()
Table 1. Reactants and spectral data of oxazino quinoline derivatives.
![]()
Table 2. Synthesis of dimethyl-dihydro-7H-chromeno [3, 2-h]quinolin-8(9H)-one derivatives.
![]()
Table 3. Comparative study of synthesis with catalysts.
other catalysts the reactions times are very much higher. Under reflux conditions, synthesis of dimethyl-dihydro-7H-chromeno [3, 2-h]quinolin-8(9H)-one derivatives without use of any catalyst has been reported. The present method offers a comparatively very low cost and easy preparation.
3.2. Plausible Mechanism for the Synthesis of Dimethyl-Dihydro-7H-Chromeno [3, 2-h]Quinolin-8(9H)-One Derivatives
In this reaction 8-hyroxy quinoline, aldehydes and dimidone are taken as reactants to run the process. Initially aromatic aldehydes undergo nucleophillic addition with 8-hydroxy quinoline through knovenegal condensation mechanism to form the intermediate Knovenegal product (1). In the second step the dimidone undergo enolisation. The formed enol product reacts with knovenegal product to form the highly stabilized product dimethyl-dihydro-7H-chromeno [3, 2-h]quinolin-8(9H)-one derivatives smoothly shown in Scheme 2.
4. Biological Activity
The antibiotic potency can be determined using the microbial assays. The basic principle of microbial assay lies in comparison of the inhibition of growth of bacteria by measuring concentration of the product to be investigated with that produced by known concentration of the antibiotic having a known activity.
The methods used for assay are cup plate method and disc diffusion method. The cup plate method is based on the diffusion of an antibiotic from a cavity
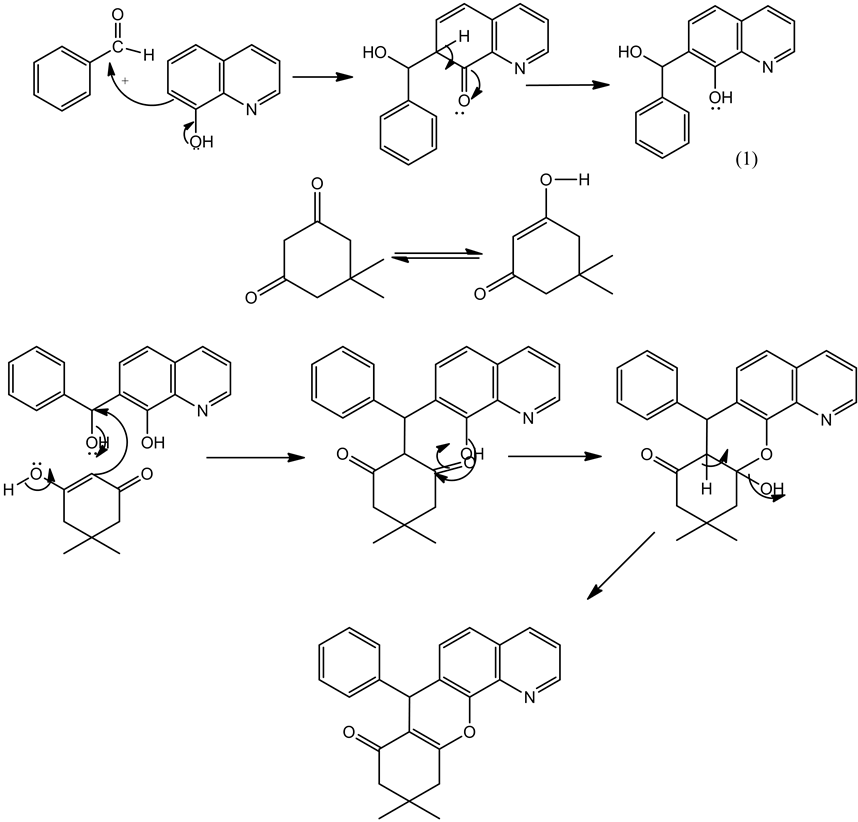
Scheme 2. Plausible mechanism of dimethyl-dihydro-7H-chromeno[3, 2-h]quinolin-8(9H)-one derivatives.
through the solidified agar layer of a Petri-dish. Growth of inoculated microbe is inhibited entirely in a circular zone around a cavity containing a solution of the antibiotics. Antimicrobial activity of synthesized compounds was screened against four human pathogenic bacteria, two gram positive and two gram negative bacteria and their respective MTCCNO numbers are given in parenthesis as Escherichia coli (Gram ?ve)-(2692), Pseudomonas aeruginosa (Gram ?ve)- (2453), Staphylococcus aureus (Gram +ve)-(902), Bacillus subtilis (Gram +ve)- (441). The activities of the drug samples against 4 human pathogenic bacteria are tabulated in Table 4.
The antibacterial activity of the samples is assessed using the different concentration of the sample i.e., low, intermediate, high.
The present investigation reveals that the zone of inhibition increased as the concentration of the sample increased. This is seen in case of the compounds 4a and 4e, 4h Hence the MIC (Minimum Inhibitory Concentration) of these samples that can inhibit bacterial growth is 10 µl, 20 µl and 30 µl respectively. Thus the above samples are able to show antibacterial activity on Escherichia coli,
![]()
Table 4. Antibacterial activities of drug samples.
Pseudomonas aeruginosa, Staphylococcus aureus, Bacillus subtilis.
The standard drug streptomycin is found to be very effective anti-microbial agent. Here it is found that the standard drug show antibacterial activity on both Gram +ve and ?ve bacteria and it is found that the zone of inhibition increased as the concentration of the sample increased.
5. Conclusion
In this present study, we report an efficient greener method for the synthesis of dimethyl-dihydro-7H-chromeno[3, 2-h]quinolin-8(9H)-one derivatives. This method has several advantages like improved yield of products, less reaction times.