Elaboration and Characterization of Glasses and Ceramic-Glasses within Theternary Diagram Li2O-Cr2O3-P2O5 ()
1. Introduction
Several fundamental studies have been made on glasses composed of silica and with oxides of phosphoric anhydrides [1] [2] [3] [4] . Due to their low chemical durability, phosphate glasses have gained less attention. However, several phosphate glasses with high aqueous corrosion resistance have been reported [5] - [12] . The electrical engineering field constitutes one of the numerous fields of application of glasses. This field is particularly interested in materials with high ionic conductivity that can be used as solid electrolytes, or in materials of very high resistivity capable of playing the dielectric role for miniaturised condensers. Historically, ionic conduction has been studied in crystalline compounds for a long time [13] . The interest in ion-conducting glasses is more recent, but has progressed rapidly over the last thirty years due to the use of these materials as solid electrolytes. The transport properties of these electrolytes have been the subject of numerous works, whose main lines have been grouped together in some publications [14] [15] [16] [17] . The notion of point defects is a very useful tool in the interpretation of conduction in crystallised materials, but it is ill-defined for amorphous materials, because they do not have long-range structural periodicity. However, glasses have many spaces (due to their low density), which are capable of containing cations of different sizes [7] [16] [18] [19] . Ions that are weakly bonded to the network can move in the material under the action of a force resulting from an electric potential gradient. The well-known mobility of alkaline cations, and in particular Li+ and Na+, in solid electrolytes has prompted us to explore the electrical conductivity of the revealed vitreous phases [2] [3] [4] [5] . Our aim in the present work is to define the glass area in the ternary system Li2O-P2O5-Cr2O3 for the first time. A second aim is to undertake an electrical study of glasses and glass ceramics of the ternary system Li2O-P2O5- Cr2O3. The study of the conduction mechanisms and their relation to the structure of the phosphorus network illustrate that with increased amounts of the modifier oxide Li2O in the glass network, the mobility of the Li+ carriers is facilitated, due to increased depolymerisation of the phosphate network [7] [18] [19] . On the other hand, the increasing glassy depolymerisation of the network induces an increase in the number of non-bridge oxygens and consequently an increase in the dielectric permittivity [7] [18] [20] [21] .
2. Experimental Procedures
A glass of composition xLi2O-yCr2O3-(100-(x + y)P2O5 (mol %) was obtained by the melting quench method at 1000˚C. Appropriate mixtures of the compounds Li2CO3, Cr2O3 and (NH4)2HPO4 were initially prepared at various temperatures between 300˚C - 500˚C in order to achieve a preliminary preparation before the final glass preparation. The melts were performed in alumina crucibles for about 20 min at 1000˚C ± 10˚C. The isolated glass samples were approximately 10 mm diameter and 1 to 3 mm in thickness. Their vitreous state was first evidenced by their shiny aspect and confirmed by XRD. Annealing of these glasses was realised at increasing temperatures in intervals of 100˚C. The first structural approach was X-ray diffraction, which allowed the crystallisation of the vitreous domain Li2O-P2O5-Cr2O3 to be followed. The dielectric permittivity (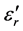 ) and dielectric loss (tnδ) measurements were determined from capacity measurements realised on samples in the form of small cylinders carved with abrasive papers. While depositing a layer of silver lacque on the parallel faces of the samples, we form a condenser plan of which the capacity is measured using a Hewlett Packard 4262 A LCR Meter. The measures of conductivity were determined from resistivity measurements realised on samples. The samples were polished in the form of small cylinders using abrasive papers. Pastilles so obtained have diameters of around 10 mm and thicknesses varying between 1 and 3 mm.
) and dielectric loss (tnδ) measurements were determined from capacity measurements realised on samples in the form of small cylinders carved with abrasive papers. While depositing a layer of silver lacque on the parallel faces of the samples, we form a condenser plan of which the capacity is measured using a Hewlett Packard 4262 A LCR Meter. The measures of conductivity were determined from resistivity measurements realised on samples. The samples were polished in the form of small cylinders using abrasive papers. Pastilles so obtained have diameters of around 10 mm and thicknesses varying between 1 and 3 mm.
3. Results
In the Li2O-P2O5 system, transparent and colourless glasses could be prepared with molar fractions of Li2O between 0 and 0.62. These values are in good agreement with previously published results [18] but are rather superior to those given by the other authors [20] [22] . This is probably caused by different experimental conditions (temperature of melting; speed of tempering, etc.) [23] . In the binary systemCr2O3-P2O5, the substitution of P2O5 by Cr2O3 led to very hygroscopic green glasses with a chromium oxide content between 0 and 7 mol%, see Figure 1. Ternary glasses have also a green colour which becomes more clear and green as the lithium oxide content increases and as the Cr2O3 content increases between 0 £ y < 5; (mol%). The demarcation of the glassy zone within the ternary diagramLi2O-P2O5-Cr2O3 is given by the following limits: 0 £ x £ 62; 0 £ y <7; 36 £ χ £ 100 (mol%) (Figure 1). The localization of analysed compounds is presented inside the ternary diagram given in Figure 2.
3.1. Annealing Temperature
The annealing temperatures of the binary Li2O-P2O5, ternaries glasses and ceramic glasses were tested at increasing temperatures in intervals of 100˚C. Increasing amounts of the lithium oxide modifier lead to an increase of the values for the crystallisation temperature. For the glasses and ceramic glasses of composition xLi2O-2Cr2O3-(98-χ)P2O5 and xLi2O-5Cr2O3-(98-χ) P2O5, Figure 3 shows that the crystallisation temperature increases from (250 ± 10)˚C to (540 ±
![]()
Figure 1. Extended from the vitreous field within the ternary diagram Li2O-P2O5-Cr2O3 to 1000˚C.
![]()
Figure 2. Localization of the investigatedglass and ceramic glass compositionsin the ternary diagram Li2O-Cr2O3-P2O5.
![]()
Figure 3. Variation of crystallization temperature as a function of the Li2O/P2O5 ratio for glasses and ceramic glasses with the composition Li2O-Cr2O3-P2O5.
10)˚C as the Li2O/P2O5 ratio increases from 0 to 2. However, the annealing temperature (Tc) variations for the ceramic glasses are greater than those observed in the glasses when the Li2O content increases.
3.2. Electrical Conductivity
The electrical conductivities were measured at various temperatures between 25˚C and 300˚C. Figure 4 shows the variation of the logarithm of the conductivity as a function of the inverse of the absolute temperature for some compositions. In a large part of the envisaged domain of temperature, the conductivity σ varies according to Arrhenius’s law σ0 Exp Ea/kT where σ0 is the pre-exponential term, Ea is the activation energy of conduction, and k is the Boltzmann constant, 1.38 × 10−23 J/K. The activation energies, Ea, calculated from this relationship are shown in Table 1 as are the conductivities at 300˚C. Examination of this table shows that the activation energy is a decreasing function of the Li2O content. Figure 5 clearly indicates the variations of the conductivity at 300˚C according to the Li2O content for the glasses and glass ceramics with different Cr2O3 contents. We can conclude that the introduction of chromium oxide into the phosphate network seems to change the behaviour from that of a glass to that of a ceramic glass. We note a decrease of σ for glasses, whereas we observe an increase of σ for ceramic glasses. On the other hand Figure 6 indicates that the
![]()
Figure 4. The Arrhenius plot for some glasses and glass-ceramics, (mol%) in the ternary diagram Li2O-Cr2O3-P2O5.
![]()
Table 1. Glasses and ceramic glasses composition in mol% and selected properties as electrical conductivity and activation energies.
![]()
Figure 5. Variation of the conductivity logarithm at 300˚C as a function of Li2O content for some glasses and ceramic glasses in the ternary diagram Li2O-Cr2O3-P2O5.
![]()
Figure 6. Variation of the activation energy at 300˚C as a function of Li2O content for some glasses and ceramic glasses in the ternary diagram Li2O-Cr2O3-P2O5.
![]()
Figure 7. Variation of dielectric permittivity with temperature at 10 KHz of binary glasses with the composition x Li2O-(100-x)P2O5.
activation energies, for different lines, varies inversely proportionnel with the increase of the Li2O content [7] [18] [20] [24] .
3.3. Dielectric Permittivity
Figures 7-9 show the thermal variations of log 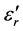 for three families of composition: xLi2O?(100-x)P2O5; xLi2O-2Cr2O3?(98-x)P2O5; and xLi2O-5Cr2O3-(95-x) P2O5. Examination of these figures shows that when the temperature and the composition are modified, the variation in the dielectric values is proportionally greater than those of silicate glasses [1] [2] [3] . However the values of
for three families of composition: xLi2O?(100-x)P2O5; xLi2O-2Cr2O3?(98-x)P2O5; and xLi2O-5Cr2O3-(95-x) P2O5. Examination of these figures shows that when the temperature and the composition are modified, the variation in the dielectric values is proportionally greater than those of silicate glasses [1] [2] [3] . However the values of 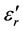 remain very modest below a certain temperature (Tt) after which the log
remain very modest below a certain temperature (Tt) after which the log 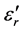 slope increases more and more quickly with increased Li2O content. Figure 10 shows that the curve (
slope increases more and more quickly with increased Li2O content. Figure 10 shows that the curve (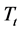 ) changes according to the Li2O content
) changes according to the Li2O content 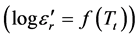 for all different lines studied, manifested as an increase in
for all different lines studied, manifested as an increase in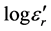 , and a negative slope. The results are regrouped in Table 2. The study of the
, and a negative slope. The results are regrouped in Table 2. The study of the 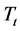 in relation to the Li2O content shows that when the Li2O content increases for various compositions, the temperature decreases. Similar results have been shown in previous studies [18] [20] .
in relation to the Li2O content shows that when the Li2O content increases for various compositions, the temperature decreases. Similar results have been shown in previous studies [18] [20] .
3.4. Dielectric Loss
Figures 11-13 show the curves representing thermal variations of the dielectric
![]()
Figure 8. Variation of dielectric permittivity with temperature at 10 KHz for glasses with the composition -xLi2O-2Cr2O3(98-x)P2O5.
![]()
Figure 9. Variation of dielectric permittivity with temperature at 10 KHz for ceramic glasses with the composition xLi2O-5Cr2O3-(95-x)P2O5.
![]()
Figure 10. Variation of Tt as a function of the Li2O content for glasses and ceramic glasses with the composition x Li2O-yCr2O3-(100-(x + y))P2O5.
![]()
Table 2. Glasses and ceramic glasses composition in mol% and selected properties as Permittivity and Tt.
![]()
Figure 11. Thermal variation of dielectric losses at 10Khz of glasses with the composition xLi2O-(100-x)P2O5.
losses of glasses and vitreous ceramics of the ternary system Li2O-P2O5-Cr2O3. These curves show a more or less Gaussian behaviour with maxima of tnδ, which appear at decreasing temperatures as the Li2O content increases. The temperatures of these maxima seem to coincide with the Tt observed on the log![]() (Tt) curves. Moreover, Figure 14 shows that the increase in Cr2O3 content in the ulraphosphate units (50 £ χ £ 100; mol%) does not seem to have a notable
(Tt) curves. Moreover, Figure 14 shows that the increase in Cr2O3 content in the ulraphosphate units (50 £ χ £ 100; mol%) does not seem to have a notable
![]()
Figure 12. Thermal variation of dielectric losses at 10 Khz of ceramic glasses with the composition xLi2O-2Cr2O3-(98-x)P2O5.
![]()
Figure 13. Thermal variation of dielectric losses at 10Khz of ceramic glasses with the composition Li2O-5Cr2O3-(95-x)P2O5.
![]()
Figure 14. Thermal variation of dielectric losses at 10 Khz of glasses and ceramic glasses with the composition (60-y)Li2O-yCr2O3-40P2O5.
influence on the position of the maxima of tnδ (T) for the glasses and the vitreous ceramics, but specially induces the decrease of the amplitude of the dielectric losses.
3.5. Infrared Spectroscopy
The infrared spectra of the glasses of the binary system Li2O-P2O5 are shown in Figure 15. Increase of the lithium oxide content in the phosphate glasses causes an important depolymerisation in the vitreous network, and induces the formation of small units in accordance with the results of M. Ouchetto et al. [18] [19] [25] . The analysis of infra-red spectra shows that the band at 455 - 500 cm−1 is assigned to the deformation of the skeleton δ ske (P-O-P while the band at 735 - 775 cm−1 and the bands at 900 - 910 cm−1 are assigned respectively to the vibration of symmetric stretching νsym (P-O-P and to the asymmetric stretching νasym (P-O-P) [9] [11] . The band υasPO2 that appears about 1250 - 1275 cm−1, attributed to Q2 phosphate tetrahedron, decreases while we move away from point P2O5. Whereas the band υsPO2 that appears about 1090 - 1100 cm−1attributed to Q1 phosphate tetrahedron, increases with the increasing of Li2O content and inducts a break links P-O-P and an increase of the number of non bridge-oxygen. In the other hand, the IR spectra with the compositions xLi2O-2Cr2O3?(98-x)P2O5 and xLi2O-5Cr2O3-(95-x)P2O5, observed in the Figure 16 and Figure 17 respectively, have similar behaviours to the spectra of the binary system xLi2O-(100-x) P2O5 family, which can be explained by a similar structural evolution [7] . We note the presence of a small band uas P-O-P at 995 - 998 cm−1, attributed to isolated orthophosphate units, when the Li2O content exceeds 55 mol%. This last one can be attributed to the Cr?O?P bond in CrPO4 units [26] . Also, the band υsP = 0 that appears about 1360 cm−1 diminishes while on moves away from point P2O5, that seems to be attributed to the basic glass matrix P2O5 [18] .
![]()
Figure 15. I.R spectra of phosphate glasses with the composition xLi2O-(100-x)P2O5.
![]()
Figure 16. I.R spectra of chromium phosphate glasses with the composition xLi2O- 2Cr2O3-(98-x)P2O5.
![]()
Figure 17. I.R spectra of chromium phosphate ceramic glasses with the composition xLi2O-5Cr2O3-(95-x)P2O5.
4. Discussion
The regular melting-quench method allowed the isolation of a small vitreous domain within the ternary system Li2O-P2O5-Cr2O3 at 1000˚C. Conductivity measurements were performed on glasses and vitreous ceramics isolated within the ternary systemLi2O-P2O5-Cr2O3. Dielectric permittivity and dielectric loss were measured at a frequency of 10 kHz and in the temperature range of 298 to 573 K. An increase in the Li2O content in the glass network results at the same time in an increase in ![]() (T) and an increase in tgδ. These phenomena result from a increasing depolymerisation of the glass network due to increased numbers of non-bridge oxygens because of the progressive breaking of phosphorus network links as the modifier Li2O content increases [7] [18] [21] . Indeed, the oxide modifier Li2O has much lower link energies than those of the formative oxides [24] . As a consequence the mobile lithium ion is weakly connected to the network and will be thermally activated to cross the barriers of potential presented by the network, involving the formation of non-bridge oxygens by the breaking of P-O-P links. This leads to the formation of the - P - O- entities, which are easily polarised, hence the increase in
(T) and an increase in tgδ. These phenomena result from a increasing depolymerisation of the glass network due to increased numbers of non-bridge oxygens because of the progressive breaking of phosphorus network links as the modifier Li2O content increases [7] [18] [21] . Indeed, the oxide modifier Li2O has much lower link energies than those of the formative oxides [24] . As a consequence the mobile lithium ion is weakly connected to the network and will be thermally activated to cross the barriers of potential presented by the network, involving the formation of non-bridge oxygens by the breaking of P-O-P links. This leads to the formation of the - P - O- entities, which are easily polarised, hence the increase in![]() . On the other hand, more the concentration of the modifier oxide increases, the greater the number of lithium-oxygen-phosphorus bonds in the vitreous network more the calorific energy needed to break the potential barriers presented by the network is low, hence, temperature (Tt) decreases. However, an increase in chromium oxide content, even at very low concentration, induces a decrease of the dielectric losses and an increase of the Tt temperature. Hence we can say that an increase in Cr2O3 content induces an increase of the rigidity of the glass network. However, this behaviour changes when the system changes from a glass to a ceramic glass. The study of the conductivity as a function of the temperature for various samples shows that σ varies according to Arrhenius’s law. Increase in temperature andLi2O content both induce an important increase in the conductivity. The mobile lithium ion (Li+) is weakly connected to the network and will be thermally activated to cross the potential barriers presented by the network. This leads to the initiation of greater ionic conduction as the number of free Li+ ions increases. The effects of Cr2O3 on the conductivity and the activation energy in glasses and ceramic glasses are different. We note that the conductivity decreases in the glasses followed by an increase of the activation energy, while the conductivity increases in vitreous ceramics followed by a decrease of the activation energy. However the decrease of σ in the chromium glasses can be explained by the replacement of Li-O-P or P-O-P hydrated bonds by Cr-O-P covalent bonds. Cr3+ ions would be trapped in sites of low coordination (£6) and so would be a part of the formative network [27] . The glassy domain bounded in our experimental conditions (Tf = 1000˚C) is relatively small and the vitreous ceramics studied have a composition rather close to the border area separating glassy and crystallised domains inside the ternary structure Li2O-P2O5-Cr2O3. Thus, the network defects are probably more numerous there and in a favourable position with regard to the mobile species. We can conclude that the conductivity seems to improve when we pass from the glassy domain to the vitreous ceramic domain [28] . At the same time, the increased Li2O content in the vitreous network and increased temperature, both induce an increase in the dielectric permittivity. Mean while, the progress of Tt seems to exhibit different behaviour when we change from glasses to glass ceramics. This could also be explained by the border area separating the glassy and crystallised domains.
. On the other hand, more the concentration of the modifier oxide increases, the greater the number of lithium-oxygen-phosphorus bonds in the vitreous network more the calorific energy needed to break the potential barriers presented by the network is low, hence, temperature (Tt) decreases. However, an increase in chromium oxide content, even at very low concentration, induces a decrease of the dielectric losses and an increase of the Tt temperature. Hence we can say that an increase in Cr2O3 content induces an increase of the rigidity of the glass network. However, this behaviour changes when the system changes from a glass to a ceramic glass. The study of the conductivity as a function of the temperature for various samples shows that σ varies according to Arrhenius’s law. Increase in temperature andLi2O content both induce an important increase in the conductivity. The mobile lithium ion (Li+) is weakly connected to the network and will be thermally activated to cross the potential barriers presented by the network. This leads to the initiation of greater ionic conduction as the number of free Li+ ions increases. The effects of Cr2O3 on the conductivity and the activation energy in glasses and ceramic glasses are different. We note that the conductivity decreases in the glasses followed by an increase of the activation energy, while the conductivity increases in vitreous ceramics followed by a decrease of the activation energy. However the decrease of σ in the chromium glasses can be explained by the replacement of Li-O-P or P-O-P hydrated bonds by Cr-O-P covalent bonds. Cr3+ ions would be trapped in sites of low coordination (£6) and so would be a part of the formative network [27] . The glassy domain bounded in our experimental conditions (Tf = 1000˚C) is relatively small and the vitreous ceramics studied have a composition rather close to the border area separating glassy and crystallised domains inside the ternary structure Li2O-P2O5-Cr2O3. Thus, the network defects are probably more numerous there and in a favourable position with regard to the mobile species. We can conclude that the conductivity seems to improve when we pass from the glassy domain to the vitreous ceramic domain [28] . At the same time, the increased Li2O content in the vitreous network and increased temperature, both induce an increase in the dielectric permittivity. Mean while, the progress of Tt seems to exhibit different behaviour when we change from glasses to glass ceramics. This could also be explained by the border area separating the glassy and crystallised domains.
5. Conclusions
The regular melting-quench method allowed the isolation of a small vitreous domain within the ternary system Li2O-P2O5-Cr2O3 at 1000˚C. Conductivity measurements were realised on glasses and vitreous ceramics isolated within the ternary system Li2O-P2O5-Cr2O3. Dielectric permittivity and dielectric loss were measured at a frequency of 10 MHz and in the temperature range 298 to 573 K. An increase in the Li2O content in the glass network results at the same time in an increase in ![]() (T) and an increase in tgδ. These phenomena result from an increasing depolymerisation of the glass network due to increased numbers of non-bridge oxygens because of the progressive breaking of phosphorus network links as the modifier Li2O content increases. Conductivity measurements were realised on glasses and ceramic ceramics isolated within the ternary system Li2O-P2O5-Cr2O3. In both glasses and ceramic glasses, the value of σ increases as Li2O content increases. The increase of σ is probably induced by an increase in the number of Li+ carriers whose mobility is facilitated by the depolymerisation of the phosphorus network.
(T) and an increase in tgδ. These phenomena result from an increasing depolymerisation of the glass network due to increased numbers of non-bridge oxygens because of the progressive breaking of phosphorus network links as the modifier Li2O content increases. Conductivity measurements were realised on glasses and ceramic ceramics isolated within the ternary system Li2O-P2O5-Cr2O3. In both glasses and ceramic glasses, the value of σ increases as Li2O content increases. The increase of σ is probably induced by an increase in the number of Li+ carriers whose mobility is facilitated by the depolymerisation of the phosphorus network.
Increase in the Cr2O3 content seems to have caused different behaviour from glasses to vitreous ceramics. The conductivity decreases in the chromium glasses while it increases in glass ceramics due to network defects, which are probably more numerous and in a favorable position for mobile species. On the other hand, an increase in the chromium oxide content induces a decrease dielectric loss and an increase of Tt temperature in the glass. Hence we can say that an increase of Cr2O3 content induces an increase of the rigidity of the glass network. The highest conductivity obtained at 300˚C was in the order of 2 × 10−3 (Wcm)−1 with an activation energy of 0.45 e.v; however, this remains low compared with that of the crystalline compound LiAl11O17 (σ =10−2 (Ωcm)−1) at 25˚C. The obtained conductivities are probably responsible for large dielectric losses, which mean that our glasses are currently not suitable for possible application as dielectric condensers.