Species Diversity and Structure of an Intact Freshwater Swamp Forest in the Niger Delta ()
1. Introduction
Tree species in tropical forests are varied at different spatial scales and characterized with high diversity across the Tropics and Neotropics. This could be broadly estimated to be more than 100 species per hectare ( Gentry, 1990 ; Huang et al., 2003 ), or specifically seen to range between 56 and 283 (>10 cm DBH) in mature tropical forests ( Philips & Gentry, 1994 ) and 300 or more species of trees (≥10 cm DBH) per hectare in the Neotropics ( Gentry, 1988 ; Valencia et al., 1994 ; Huang et al., 2003 ; Kelloff, 2008 ). The dominant species have been reported to vary from place to place; from Leguminosae in Neotropical lowland forests and African lowland forests, to Dipterocarps in Southeast Asia ( Gentry, 1988 ; Whit- more, 1998 ; Huang et al., 2003 ). Though the African forests are composed of large tree biomass, its diversity has been reported to be relatively poor compared to higher diversities in Asian and American forests ( Parmentier et al., 2007 ; Chuyong et al., 2011 ; Malhi et al., 2013 ). While this could be attributed to climate (mainly annual rainfall and rainfall seasonality) ( Francis & Currie, 2003 ; Field et al., 2005 ; Parmentier et al., 2007 ), a good baseline is yet to be established for its diversity as only few systematic studies have been conducted in the region regarding the basic attributes such as biomass, species diversity and structure ( Parmentier et al., 2007 ; Chuyong et al., 2011 ; Gourlet-Fleury et al., 2013 ; Malhi et al., 2013 ; Peh et al., 2014 ). While some studies in Nigeria have enumerated the diversity and species composition of different forest sites ( Ihenyen et al., 2009 ; Ihuma et al., 2011 ; Adekunle et al., 2013 ; Ogbemudia et al., 2013 ; Udofia et al., 2014 ), studies that systematically showed the species diversity and structural baselines with particular reference to the freshwater swamp ecosystem are still much needed.
2. Materials and Methods
2.1. Study Site
The study was carried out in Otuwe forest (Figure 1)―an intact forest location in the flood forest freshwater swamp forests of the Niger Delta. This forest land is owned by the Akarai-Obodo community and accessible mainly during the dry season due to poor accessibility (no access roads or waterway connecting it to the community or elsewhere). The community as well as the forest is located in Ndokwa East Local Government Area of Delta state, Nigeria (Figure 1). The forest which is named after a lake, is found in the Niger basin of Nigeria and is made up of marine sediments of the upper and lower cretaceous age. Being
categorized broadly as hydromorphic soils ( Areola, 1982 ), the soils are characterized by both the seasonally and permanently waterlogged soils; which are uniformly alluvial. The study region is a vast sedimentary region and flat area that is criss-crossed by a large number of meandering streams and creeks. It has a tropical climate with long rainy season, relative humidity which rarely dips below 60% and an average monthly maximum and minimum temperatures varying between 28˚C to 33˚C and 21˚C to 23˚C, respectively. The flood regime of the region begins toward the end of the rainy season in August, peaks in October, and tapers off in December.
The freshwater swamp forest of the Niger Delta is the most extensive and largest across Nigeria and West Africa ( NDES, 1997 ; Spalding et al., 2010 ). It is divided into the seasonally flooded and permanently flooded zones. The ecosystem has transition zones with the lowland rainforest and mangrove ecosystems in the northern and southern parts of the ecosystem respectively. The region has been inhabited since about 5000 Years before present (YBP) ( Nzewunwa, 1985 ) and much of the forest ecosystem have been exploited for timber production, industrial activities and domestic functions, mainly as sources of firewood and charcoal. Much of the forest in this region has been converted into plantation farms and overtaken by urbanization and industrial activities―mainly crude oil exploration. The ecosystem is threatened by salt water intrusion from the canalization and crude oil prospecting activities in the region, dredging and land reclamation, and population pressure.
2.2. Vegetation Data Collection
Standard plot-based method, which involves a one-time census of all stems ≥ 10 cm in diameter using a 1 ha square shaped (Phillips et al. 2003; Newton, 2007) was employed for this work between December 2013 and April 2014. Each of the 1 hectare square plots were established on transects that were 1 km wide apart, and contained plots of 100 × 100 m each at the interval of 500 m between each of the plots. The interval between the plots and transects were to ensure that there is a sizeable distance to enable floristic variation, yet maintaining consistency. All stems ≥10 cm in diameter at breast height (130 cm) were analysed and smaller ones omitted in order to ensure that species identification had minimal bias. Stem heights was determined using a laser rangefinder and where the tree height was less than 10 meters, graduated poles was used in measuring the height. Tree species were identified in the field by a trained taxonomist from the Forestry Research Institute, Ibadan (FRIN), while the specimen for the unidentified ones were collected and verified at FRIN herbarium. Specie identification followed the taxonomy of Nigerian plants ( Keay, 1989 ).
2.3. Data Analyses
2.3.1. Forest Structure Analyses
In order to characterize the forest sites, the importance values of the species (species importance values) and the families (family importance values) were calculated after Cottam and Curtis (1956) , Mori et al. (1983) and Husch et al. (2003) as follows:
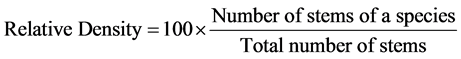 (1)
(1)
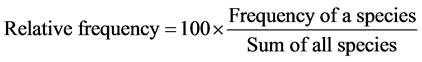 (2)
(2)
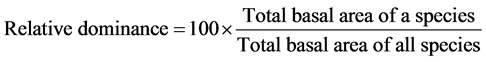 (3)
(3)
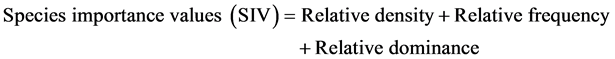 (4)
(4)
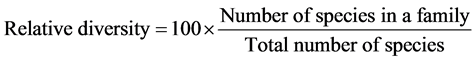 (5)
(5)
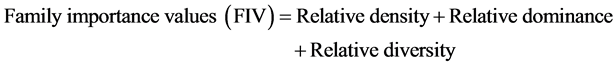 (6)
(6)
Dominance was defined as species or families with ecological values ≥ 10 for this study.
2.3.2. Basal Area
The basal area was calculated as follows:
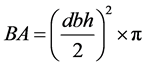 (7)
(7)
where BA is the basal area (m2); dbh is the diameter at breast height (cm) and 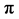 as pie (3.142).
as pie (3.142).
The stem sizes were categorized into the following forest structural analysis: small (10 - 20 cm dbh); medium (21 - 50); large (51 - 100) and largest (>100) ( Adekunle et al., 2013 ). Heights of the trees were categorized into classes using an interval of 5 (≤5, 6 - 10, 11 - 15, …) and used to plot a graph to depict the vertical structural pattern of the forest.
2.3.3. Species Diversity Analyses
The diversity indices were calculated following Kent and Coker (1992) , Magurran (1998) and Magurran (2004) as follows:
Shannon-Wiener index:
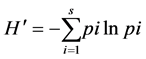 (8)
(8)
where 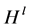 is the Shannon-Weiner index, s is the total number of species, pi is the proportion of individuals in the ith species, and ln is the natural logarithm.
is the Shannon-Weiner index, s is the total number of species, pi is the proportion of individuals in the ith species, and ln is the natural logarithm.
Pielou’s evenness index:
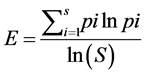 (9)
(9)
Margalef’s species richness index:
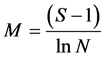 (10)
(10)
where S is the total number of species in a community, N is the number of individuals and ln is the natural logarithm.
Pearson correlation was used to show the relationship that existed among the biodiversity indices using IBM SPSS software version 20.
3. Results
3.1. Floristic Composition, Abundance and Dominance
This forest ecosystem is composed of 35 species within 28 genera and 18 families. The number of species found across the 8 plots in the forest site ranged from its lowest of 4 species found in plot 3 to the highest number (19) found in plot 4. The average abundance of the species across the forest was concentrated on four species: Celtis zenkeri Engl, Diospyros mespiliformis Hochst, Sterculia oblonga Mast and Sterculia rhinopetala K. Schum; which contributed 92.89% (Table 1) of the total species occurrence, while the remaining 7.10% were from the other 31 species in the forest. Among the 35 species present in the forest, the dominant species across the forest site were: Diospyros mespiliformis, Sterculia oblonga, Sterculia rhinopetala, Celtis zenkeri Engl and Erythrophleum ivorense A. Chev, with species importance values of 83.61, 66.29, 35.51, 26.32 and 13.31 respectively. The dominant families (family importance value) across the forest site were: Malvaceae, Ebenaceae, Leguminosae and Cannabaceae, with importance values of 106.93, 72.36, 40.51 and 20.75 respectively.
3.2. Species Diversity and Rarity
The species found in the ecosystem had a total of 2043 (stems) occurrences across the 8 plots. The pattern of species occurrence varied from the species that had > 100 stems (14.28%), >1 < 100 stems (62.85%), and those with only a single stem occurrence (22.85%). Leguminosae had the highest number (9) of species and was followed by Malvaceae (5); Moraceae (3); Annonaceae, Apocynaceae, Ebenaceae and Olacaceae (2 species each), while the remaining families were represented by one species each (Table 2).
The species diversity across the site was low: with the highest species diversity (2.13) seen in plot 1 and the least diversity of 0.98 in plot 3; and a mean value of 1.66 (Table 3). Pielou’s evenness index ranged from 0.83 to 0.55 (Table 3), while the mean value across the site was 0.72. Margalef’s species richness index ranged
![]()
Table 1. Summary of the abundance and characterizing species across the sites.
![]()
Table 2. Number of species per family across the forest site.
![]()
Table 3. Biodiversity indices across the forest site.
from 0.52 to 3.26; with a mean value of 1.85. Mean number of species recorded across the plots was 11 (which ranged from 4 to 19 species) (Table 3).
The pattern of species rarity across the forest showed the highest value of 7 single (rare) species and a no occurrence value (0) as its least. On the average, the pattern of rarity across the forest site was low (2.62).
The results of the Pearson correlation coefficient (significant at 0.05 levels) showed that species richness correlated with species diversity and rarity. However, the highest correlation (r value) of 0.78 was between species richness and species rarity, an average correlation of 0.48 between species richness and diversity, and no significance between species richness and evenness.
3.3. Forest Structure
The highest total basal area of 30,995.62 m2 was contributed by Sterculia oblonga, followed by Diospyros mespiliformis (29,214.11 m2) and Sterculia rhinopetala (10,397.52 m2), respectively. The least basal area per hectare (0.0086 m2) was contributed by Elaeis guineensis, while the highest basal area (stem size) across the forest was 448 cm (as seen in Celtis zenkeri).
Ebenaceae had the highest relative density (38.17) across the forest and was followed by Malvaceae (29.98). However, Malvaceae had the highest total basal area of 79,107.98 m2 and was followed by Ebenaceae with 34,419.65 m2. Annonaceae had the highest mean dbh of 118.9 cm, followed by Malvaceae (55.79 cm), while Arecaceae had the least mean dbh (10.5 cm). Sterculia oblonga had the highest dbh (dominant dbh) of 19,870.8 cm, while Elaeis gunieensis and Millettia thonningii had the least dbh (10.5 cm). Diospyros mespiliformis had the highest mean height of 837.81 m, the tallest single tree in the forest was 82.5 m (Cleistopholis patens Engl. & Diels) and the mean height for the entire forest was 15.61 m.
The stem height intervals had variations in the number of stems per class, as expected in mature forests. While stem heights between 11 - 15 cm recorded the highest number of stems (662), the least value of 2 was found in the highest stem interval (≥46 cm) (Figure 2). The highest proportion (54.63%) of the trees in the forest occupied the middle stratum (11 - 25 cm), followed by the lower stratum (≤10 cm) (30.76%) and upper stratum (26 - 40 cm) (13.76%). The emergent layer (>40 cm) occupied the least value (0.83%) (Figure 2).
![]()
Figure 2. Height intervals of individual stems per hectare.
![]()
Figure 3. Diameter class of trees in the forest.
The pattern of distribution of the basal area of the forest had the highest values (dbh) from the lower class to the higher ones. The highest numbers of stems (1279) in the forest were found in dbh class of 10 - 20 cm, and reduced as the dbh classes increased consistently until the 91 - 100 cm dbh class (Figure 3). It however, experienced another increase in the number of stems for the > 100 cm dbh class (Figure 3). The number of stems in the forest was inversely proportional to the diameter sizes, shown by the reverse J (curve) pattern of the dbh interval distribution (Figure 3). This showed that the forest had a balanced species regeneration and recruitment.
4. Discussion
The freshwater swamp forest ecosystem in the Niger Delta is an ecologically important biome with unique biodiversity of regional and local importance. However, unlike other tropical forest ecosystems with higher species richness such as the Neotropics which records as much as 300 species per hectare ( Gentry, 1988 ) and other African forests where the species richness is even as low as 60 species per hectare ( Bernhard-reversat et al., 1978 ); it is much lower. Hence, while the forest recorded as much as 35 species across the entire forest plots, its abundance and dominance is concentrated only on four species. Though its mean species richness is far below other forest ecosystems both in Africa and across the Amazonia ( Gentry, 1988 ; Phillips & Gentry, 1994 ; Richards, 1996 ; Ndah et al., 2013 ), it is similar with other freshwater swamp forests which are taken to be species poor as well ( Scarano et al., 1997 ; Kurtz et al., 2013 ). While this could be generally attributed to the variations that exist in their abiotic environment, history and biogeography ( Orians et al., 1996 ), it is equally as a result of the environmental constraints associated with the ecosystem. On the other hand, the diversity of the forest ecosystem was equally low, with a mean value that is far lesser than other tropical forest ecosystems ( Knight 1975 ; Duran et al., 2006 ). This low diversity across the ecosystem, as in other swamp forests across the tropics, is not only due to the oxygen stress that plants in such flooded terrains go through, but also due to the fragmented nature of such landscapes ( van Andel, 2003 ). Even though such disturbances and fragmentation of the ecosystem are expected to enable the gap dynamics that would facilitate a higher diversity across the landscape, the rare species that eventually thrive in the ecosystem are limited and quite few. This understanding that new species recruitment and establishment is constrained by the environment is not only beneficial for establishing guidelines and restraints targeted at protecting and conserving rare species, but also useful in promoting the functional diversity of the ecosystem.
The forest structure of the ecosystem was mainly influenced by the dominant species which had the higher basal area and relative density. With a high evenness, though with a low diversity, the forest’s community structure was able to attain a mature and stable status; evidenced by the inverse J-shape. The forest had representative tree stands for each of the structural intervals or categories and a healthy juvenile/middle class tree population size (Figure 3), which is suitable for good recruitment and replacement for the mature and emergent populations. Such mature forests’ with large trees are veritable assets in carbon sequestration for the region ( Igu & Marchant, 2016 ) and useful in providing necessary ecosystem services that support the livelihood of its owners. Tree stems which ranged from 128 - 409 stems per hectare (with a mean of 255) across the forest plots were within the range of 245 - 467 stems per hectare, as reported for other tropical forests ( Campbell et al., 1992 ). Though the mean number of tree stems per hectare was less than the average numbers of 323 trees per hectare ( Aigbe et al., 2014 ), 387 stems per hectare ( Adekunle et al., 2013 ) in tropical forest reserves in Nigeria, it was seen to exhibit an adequate population structure that had both a regenerating and climax population. Its lower tree stem numbers however, is attributed to the disturbances associated with the ecosystem (such as flooding) as well as the biogeography (shallow soil depth) of the region; which promotes tree falls and uprooting of tree stems across the forest ecosystem. Relative density varied across the ecosystem as would in mature forest ecosystems, according to the different families represented in the forest. Ebenaceae had the highest relative density among the families in the forest through its single genus Diospyros (and two species―mespiliformis and crassiflora). With other families such as Malvaceae and Leguminosae with five and nine species respectively, it shows that higher species number does not necessarily translate to a higher relative density in forest ecosystems. On the other hand, while it is likely that a higher frequency of occurrence of species, genus and family across an area (relative density) would translate to a higher total basal area, however, basal area of individual large trees which may be a bit fewer in number (as in the case of Malvaceae) are more likely to record (as much as double the size) a higher cumulative basal area.
5. Conclusion
Species diversity across the ecosystem was found to be low as in other freshwater ecosystems across the globe. As this is mainly as a result of the environmental constraints associated with the swamp (oxygen stress) which limits reproduction and adaptability of most of the plants, caution needs to be applied as it concerns logging in the ecosystem, so that the ecosystem will not become bare of trees or turned to shrubs. Ensuring that only mature trees are logged will help to guarantee a balanced regeneration and recruitment process across the ecosystem, the environmental constraints and low diversity notwithstanding. Such measures are equally needed across other tropical forest ecosystems which are lost at astronomical rates, so as to ensure that such landscapes are preserved and that its species do not become extinct.