Synthesis and Biological Activity Study of Novel N1-(4-Hydroxy Benzoyl)-3-Methyl-5-Phenyl-4(N-4-Chlorophenylazo)-1,2-Diazole and Its Derivatives ()
1. Introduction
Due to increased application of a large number of heterocyclic compounds such as pesticides, herbicides, pharmaceuticals, etc., in recent times, the development in heterocyclic chemistry has been very rapid. Intensive investigations of synthetic compounds which are in many times analogy of known pharmaceutical agents result in the development of new drugs.
The stability of the heterocyclic compounds depends on the size of the ring. The three- or four-membered rings are relatively unstable, while five- and six-mem- bered rings are highly stable. The derivatives of stable five-membered ring system containing carbon with two heteroatoms, is known as Pyrazole or (1-2-diazole) [1] .
1,2-diazole nucleus and N-substituted derivatives are an organic compound with the formula C3H3N2H. Pyrazole is a weak base, with pKb 11.5 (pKa of the conjugated acid 2.49˚C at 25˚C) [2] . Pyrazoles are also a class of compounds that have the ring C3N2 with adjacent nitrogen atoms [3] .
1,2-diazoles ligands are also helpful in investigating the metallosupramolecular chemistry of functionalised 1,2-diazole ligands by the preparation and characterisation of a range of first-row transition metal coordination polymers and discrete assemblies. To this end, twenty-six ligands containing 1,2-diazole functionality have been synthesised, twenty-one of which have not previously appeared in the coordination chemistry literature. Utilising these compounds, forty new coordination compounds have been prepared and characterised by single-crystal X-ray crystallography and other analytical techniques, and their solid-state structural features have been discussed in the search for reproducible new diazole-based synthesis for the designed synthesis of new functional materials [4] [5] .
The chemistry of pyrazole and its derivatives are particularly interesting because of their potential application in medicinal chemistry as anti-inflammatory [6] , analgesic [7] , anti-bacterial [8] , muscle relaxing [9] , antifungal [10] , antitumor [11] , antiviral [12] , antiparasitic [13] , anti-tubercular [14] and anti-insec- ticidal agents [15] .
Diuretic compounds that stimulate the excretion of water are potentially useful in many disorders including most of those exhibiting oedema such as congestive heart diseases, nephritis, toxaemia of pregnancy, premenstrual tension and hypertension and also play an important role in hypertensive patients and pulmonary congestion [16] . Diuretics like mannitol, thiazides, frusemide and ethacrynic acid are used nowadays. Among these diuretics, some have toxic effects. These synthetic diuretics typically inhibit potassium secretion and lead to potassium retention [17] . Sulpha/substituted 1,2-diazoles may serve as the alternative sources for the development of new diuretic agents due to their biological activity. Sulpha/substituted 1,2-diazoles used for the treatment of diuresis in different systems of medicine, have shown diuretic activity when tested in animal models.
The present substituted 1,2-diazoles were prepared because of its good biological activity and reported exhibiting significant antibacterial activity.
2. Materials and Methods
2.1. Instrumentation
All the glassware is of borosilicate grade. All melting points were determined in one open-end capillary tube on a liquid paraffin bath and were uncorrected. The melting point of an organic compound was determined by Thiel’s melting point apparatus. Reactions were monitored by TLC using silica gel-G plate as absorbent using a ratio of C6H6:CH3COOC2H5 (9:1). The diazotization of the appropriate sulpha drug and their coupling with reactive methylene compounds was carried out under an inert nitrogen atmosphere. The IR spectra (KBr pellets) were recorded on Perkin-Elmer 157 and Shimadzu spectrometer Fourier transforms infrared FT-IR 8010. 1H NMR was reported Bruker Avance II (300.65 MHz instrument using CDCl3 as solvent and TMS as internal standard and Chemical shift expressed in parts per million (ppm) using tetramethylsilane (TMS) as an internal standard and Elemental (C, H and N) analysis was performed on an Elementar Vario MICRO cube. The mass spectra were recorded on Jeol sx-102/PA-6000 (EI) spectrometer using ionization energy of 70 ev. Elemental analyses were performed on a Carlo Erba 106 Perkin-Elmer model 240 analyzer.
4-hydroxybenzoic acid hydrazide and all reference compounds were purchased from Aldrich Chemicals. Ethanol, sodium acetate, glacial acetic acid and all other reagents were purchased from S. D. Fine Chemicals (India). The diazotization of the appropriate sulpha drug and their coupling with reactive methylene compounds was carried out by the method reported in the reference.
2.2. Chemistry
The overall reaction for the synthesis of sulpha/substituted phenylazo-1,2-diazole is proceeded by 2 steps via the synthesis of an intermediate utilized for the construction of heterocyclic moieties by alkylation and the resulting compound is 1,3-diketones. The 1,3-diketones and β-ketoesters are well-known compounds widely employed in the synthesis of a variety of organic compounds. They are known as active methylene compounds due to the reactivity of the methylene group which is placed between two electronegative groups i.e. two carbonyl functions. These active methylene compounds on treatment with sodium metal or a strong base such as sodium ethoxide generate fairly which undergo nucleophilic substitution reactions giving 2,4-dione compound. The α-hydrogen on substitution with aromatic diazonium cations affords the corresponding azo derivatives which are converted into more stable hydrazono forms. The relevant reactions are presented in Scheme 1. The product of (Scheme 1) reaction is carried out with 4-hydroxybenzoic acid hydrazide in presence of glacial acetic acid and formed 1,2-diazoles compound (Scheme 2).
2.3. Synthesis of 1-Phenyl Butane-1,3-Dione or Alkylation: (Synthesis of Intermediate Utilized for the Construction of Heterocyclic Moieties)
1-phenylbutane-1,3-dione (3) CAS No. 93-91-4 was synthesized by the action of Ethylacetate (2) CAS No. 141-52-6, on Acetophenone (1) CAS No. 98-86-2, in the presence of sodium or sodium ethoxide (Figure 1) [18] CAS No. 141-52-6.
![]()
Figure 1. Synthesis of 1-phenyl butane-1,3-dione.
2.4. Experimental Technique for the Synthesis of 1-Phenyl Butane-1,3-Dione
Sodium ethoxide (34.0 g) obtained from sodium (11.5 g) and absolute ethanol was taken in a flask equipped with a dropping funnel and a reflux condenser and surrounded by ice. To it was added ethyl acetoacetate (200 ml) followed by acetophenone (60.0 g) at such a rate gentle refluxing continued. The contents were refluxed for 3 hrs and left overnight in an ice-box. The sodium salt so obtained was filtered, dissolved in water, and acidified with acetic acid to yield 1-phenyl butane-1,3-dione. It was recrystallised from ethanol (45.0 g, 55%) as Colorless needles, m.p. 61˚C.
2.5. Scheme 1
Synthesis of N1-4-chlorophenyl hydrazono-1-phenyl butane-1,3-dione
1-phenylbutane-1,3-dione (3) was synthesized by the reaction of Ethylacetate (2) on Acetophenone (1) in the presence of sodium or sodium ethoxide. 1,3-diketones (3) react with aromatic diazonium salts (4) in an buffer medium to yield hydrazono compounds (5) (Figure 2).
A yellow Crystalline Powder Yield 76% m.p. 123˚C, anal. Calcd C16H13N2OCl Found: N 9.31 Requires: 9.32 IR (KBr): 1527 (C=C-NH-N-) 1653 (C=O), 1592 (C=C), 3279 (N=NH2 associated), 833 (C-Cl) Cm−1, NMR (CDCl3): [δ] 2.5 (S, 3H, CH3), 6.85 - 7.50 (m, 7H, ArH), 7.60 - 7.85 Cm, 2H, C2-H and C4-, ArH, 1.50 CS, 1H, (-OH=CH-C) ppm.
2.6. Experimental Technique: (Scheme 1)
4-chloroaniline (1.63 g) was dissolved in conc. HCl acid (6 ml), water (6 ml) and cooled to 0˚C Sodium nitride was added to 4-chloroaniline hydrochloride the solution was filtered quickly and then added to a well-cooled solution of sodium acetate and 1-phenyl butane-1,3-dione (0.85 g) in ethanol (12 ml) [1] [2] . The Colored precipitate was filtered, washed, dried and crystallized from ethanol giving shining yellow crystals of N1-4-chlorophenyl hydrazono-1-phenyl butane-1,3-dione (5).
2.7. Scheme 2
Synthesis of N1-(4-hydroxybenzoyl)-3-methyl-5-phenyl-4 (N-4-chlorophenylazo)-1,2-diazole
N1-(4-hydroxybenzoyl)-3-methyl-5-phenyl-4(N-4-chlorophenylazo)-1,2-diazole (8) was synthesized by the action of 4-chlorophenyl hydrazono compound (5) and 4-hydroxybenzoic acid hydrazide (6) was refluxed in glacial acetic acid and separated out.
A red crystalline powder, mp 144˚C - 146˚C, yield 74.80%, molecular formula
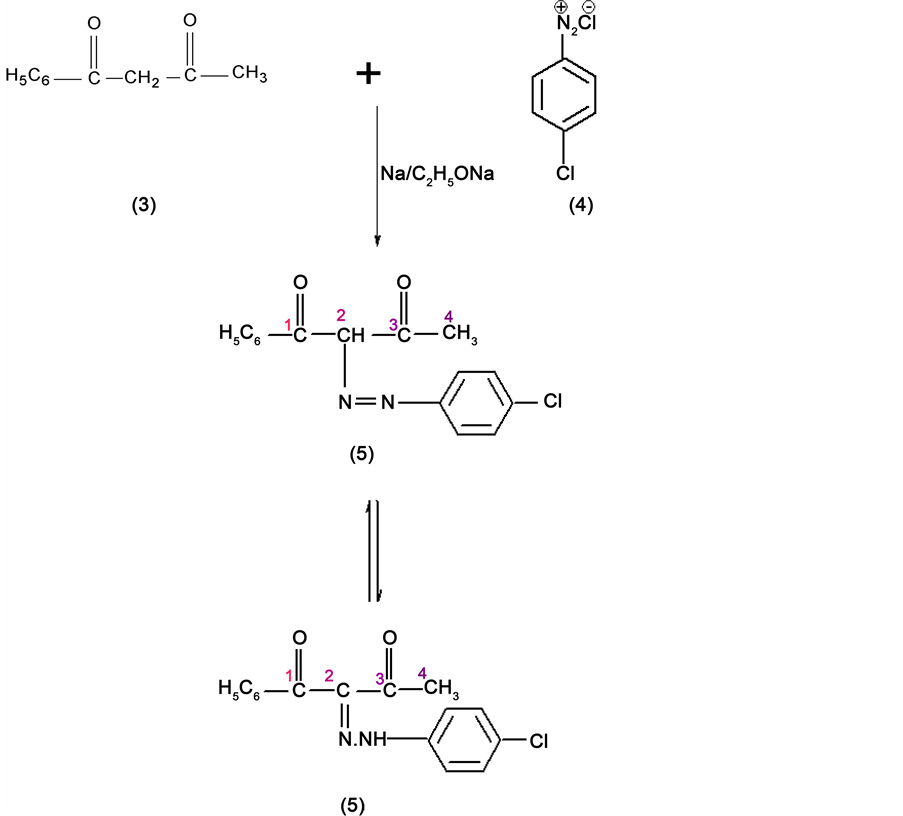
Scheme 1. Synthesis of N1-4-chlorophenyl hydrazono-1-phenyl butane-1,3-dione.
![]()
Figure 2. % inhibition of paw thickness.
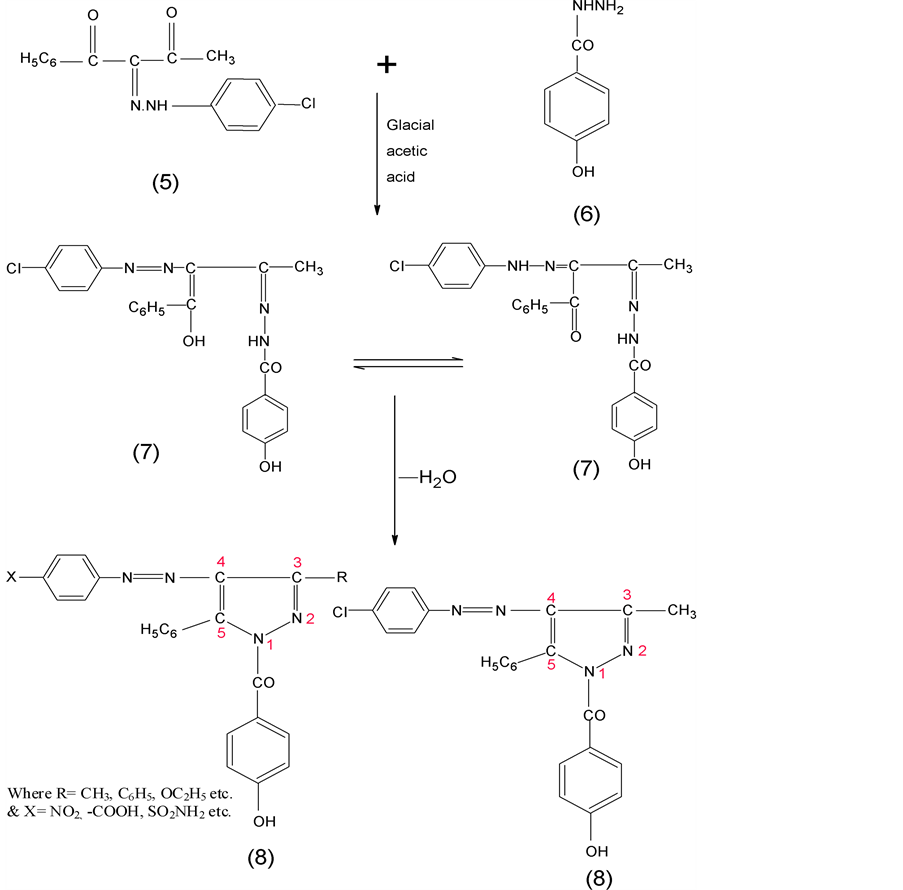
Scheme 2. Synthesis of N1(4-hydroxybenzoyl)-3-methyl-5-phenyl-4(N-4-chlorophenylazo)-1,2-diazole.
C12H14O2N4Cl, anal. Calcd. for C12H14O2N4Cl (220.50): C, 59.55; H, 4.34; O, 10.35; N, 18.12; S, 7.64. Found: C, 58.97; H, 4.64; O, 10.29; N, 16.21; IR (KBr) in cm−1 740 (C-C), 1240 (C-N), 1535 (C=C of aromatic ring), 1520 (C=N), 720 (C=Cl) 1580 (N=N), 3055 (aromatic C-H), 3135 (NH), 1707 (C=O), 3082 (NH2), 1HNMR (CDCl3) [δ] in ppm, 2.79 (s, 3H CH3), 6.65 - 7.58 (m, 13, Ar-H), 7.10 (m, 4H NH2).
2.8. Experimental Technique: (Scheme 2)
A mixture of N1-4-chlorophenyl hydrazono-1-phenyl butane-1,3-dione (5) (2.3 g) in glacial acetic acid and 4-hydroxybenzoic acid hydrazide (0.95 g) was refluxed on a water bath for about three hours and left overnight. The red colured compound was separated out, filtered, washed well with water, dried and recrystallised from ethanol and glacial acetic acid mixture to give shining red needles of title No. (8) compound [1] [2] [3] [4] .
2.9. Derivatives of Sulpha/Substituted Phenylazo-1,2-Diazoles (Table 1)
N1-(4-hydroxybenzoyl)-3-methyl-5-phenyl-4(N-4-benzylphenylazo)- 1,2-diazole (NP-1)
Steps 1 and 2 products were dissolved in glacial acetic acid and following the above general procedure desired compound was obtained in 65.33% yield; colors are Shining, Dark, Yellow, Nitrogen% found 12.12. Calculated 12.72, Rf Value 0.8457, Molecular formula C23H18O2N4, m.p. > 146˚C, IR (KBr): 1520 cm−1 (-C=N), and 3160 cm−1 (-CH-Ar), 1H NMR (CDCl3): 2.3 s (-CH2), 7.2 s (-CH=N), 3.4 s (-CH) and 5.4 - 6.8 m (Ar-H), EI-MS m/e: M+ ion peak 359.
N1-(4-hydroxybenzoyl)-3-methyl-5-phenyl-4(N-4-chlorophenylazo)-1, 2-diazole (NP-2)
m.p. 144˚C - 146˚C, yield 74.80%, molecular formula C12H14O2N4Cl, anal. Calcd. for C12H14O2N4Cl (220.50): C, 59.55; H, 4.34; O, 10.35; N, 18.12; S, 7.64. Found: C, 58.97; H, 4.64; O, 10.29; N, 16.21; IR (KBr) in Cm−1 740 (C-C), 1240 (C-N), 1535 (C=C of aromatic ring), 1520 (C=N), 720 (C=Cl) 1580 (N=N), 3055 (aromatic C-H), 3135 (NH), 1707 (C=O), 3082 (NH2), 1HNMR (CDCl3) [δ] in ppm, 2.79 (s, 3H CH3), 6.65 - 7.58 (m, 13, Ar-H), 7.10 (m, 4H NH2).
N1-(4-hydroxybenzoyl)-3-methyl-5-phenyl-4(N-4-nitrophenylazo)- 1,2-diazole (NP-3)
74.33% yield, color Pink, Yellow, Flake, Nitrogen% found 9.39, calculated 10.32, Rf Value 0.9160, molecular formula C28H19O4N5, m.p. > 270˚C, IR (KBr): 1560 cm−1 (-C=N), and 3140 cm−1 (-CH-Ar), 1H NMR (CDCl3): 2.3 s (-CH2), 6.2 s (-CH=N), 3.8 s (-CH) and 4.4 - 5.8 m (Ar-H), EI-MS m/e: M+ ion peak 365.
![]()
Table 1. Derivatives of Sulpha/Substituted phenylazo-1,2-diazoles.
N1-(4-hydroxybenzoyl)-3-methyl-5-phenyl-4(N-4-hydroxyphenylazo)- 1,2-diazole (NP-4)
65.21% yield, color Shining, Pink, Yellow, Flake, Nitrogen% found 12.00, calculated 12.18, Rf Value 0.8860, Molecular formula C28H20O4N4, m.p. > 221˚C, IR (KBr): 1460 cm−1 (-C=N), and 3150 cm−1 (-CH-Ar), 1H NMR (CDCl3): 3.3 s (-CH2), 5.8 s (-CH=N), 4.8 s (-CH) and 6.4 - 6.8 m (Ar-H), EI-MS m/e: M+ ion peak 385.
N1-(4-hydroxybenzoyl)-3-methyl-5-phenyl-4(N-4-aminodimethylphenylazo)-1,2-diazole (NP-5)
78.00% yield, color Pink, Dark, Yellow, Nitrogen% found 12.67, calculated 13.00, Rf Value 0.5023, Molecular formula C14H25O2N5, m.p. > 191˚C, IR (KBr): 1450 cm−1 (-C=N), and 3350 cm−1 (-CH-Ar), 1H NMR (CDCl3): 4.2 s (-CH2), 6.2 s (-CH=N), 6.4 s (-CH) and 8.4 - 8.8 m (Ar-H), EI-MS m/e: M+ ion peak 359.
2.10. Biological Evaluation
Animals
This study was carried out in strict accordance with the recommendations in the Guide for the care and use of Laboratory Animals of the Pasteur Institute of India, Coonoor, Tamil Nadu, India the protocol was approved by the Committee on the Ethics of Animal Experiments of the MJP Rohilkhand University, Bareilly, Uttar Pradesh, India of Permit No. RES/05/2891. All surgery was performed under Isofluorane anesthesia, and all efforts were made to minimize suffering. The adult male or female Wistar albino rats aged 2 - 3 years of either sex weighing 200 - 250 g were purchased from the Pasteur Institute of India. They were procured from National Veterinary Research centre, Bareilly, India. They were acclimated in microloan boxes with standard laboratory conditions for 7 days. The study was conducted after obtaining institutional animal ethical committee clearance. The animals were randomly allocated to six treatment groups of six animals each and kept in polypropylene cages and housed under standard conditions of temperature, humidity, dark light cycle (12 h - 12 h) and diet also [18] [19] .
The rats were randomly divided into seven groups of six animals each as follows: (I) The control group received only with saline solution. (II) Standard group received furosemide at a dose of 25 mg∙kg−1 by body weight; Groups (III), (IV), (V), (VI) and (VII) was received N1-(4-hydroxy benzoyl)-3-methyl-5- phenyl-4(N-4-chlorophenylazo)-1,2-diazole at a dose of 100 mg∙kg−1 the other derivatives by body weight and dose are represented in Table 2 respectively. Five hours prior to the experiments, the test animals were placed into metabolic cages with withdrawal of food and water [20] . After oral administration of N1-(4-hy- droxy benzoyl)-3-methyl-5-phenyl-4(N-4-chlorophenylazo)-1,2-diazole, the uri- nary output [5] of each group was recorded at different time intervals represent in Figure 3.
2.11. Anti-Inflammatory Activity
Effect of entitled (8) 1,2-diazole compound on diclofenac sodium-induced paw
![]()
Table 2. Group of animals, drugs and their dosage forms.
![]()
Table 3. Anti-inflammatory activity (Diclofenac induced paw method) of Compounds NP-1 to NP-5.
Results are expressed in mean ± SEM. (n = 6) levels of significance. *P < 0.05, **P < 0.01 and ***P < 0.001 as compared with different level of control.
edema was studied on albino Wistar rats of either sex. Test compound (100 mg/Kg body weight) and made into suspension by using 1% carboxy methyl cellulose (Vehicle) and administered through oral route. These induced paw edema is divided into seven groups of six animals and each was fasted overnight. Group I served as control and received vehicle, Group II standard (diclofenac sodium) (25 mg/Kg bw) through oral route. Group III was administered with test Compound (8) and other derivatives NP-1 to NP-5 as shown in Table 1. Test systems were kept under clinical sign observation for 30 min. the suspension of diclofenac sodium (0.1 mL of 1% w/v) was injected into the sub-planter region of right hand paw of each test system. The paw volume was measured by using digital plethysmometer (IITc Life Science, USA), immediately after injection, again at 1H, 2H, 3H and 5H intervals and results of this series against inflammation on right hand of the paw are presented in Table 3 and plotted graphically in Figure 3.
2.12. Anti Proliferative Studies
The HePG2 and EAT cell lines were grown in RPMI 1640 medium containing 10% fetal bovine serum and 2 mm L-glutamine. Compound (8) was evaluated for planter side of the left hind paw cytotoxicity against cell lines [21] [22] . The absorbance was measured at 570 mm [23] . The paw is marked with ink at the level of the lateral malleolus and immersed in mercury up to this mark. The volume of paw was measured by plethysmometer after injected, again after 1 hrs, 2 hrs, 3 hrs and 5 hrs and the percentage of cell grown inhibition was calculated using the following a formulae and absorbance are expressed in Figure 2.
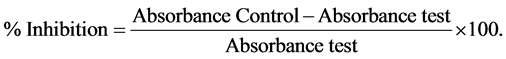
The experiments were carried at in triplicates and to the average values were plotted graphically in Figure 3.
2.13. In Vitro Antibacterial Activity
N1-(4-hydroxy benzoyl)-3-methyl-5-phenyl-4(N-4-chlorophenylazo)-1,2-diazole and other series experimented against Gram positive Staphylococcus aureus (NCIM-5022) and Gram-negative Escherichia coli (NCIM-5051) bacteria strains which were arranged from CSIR-(NCL) Pune. These anti-bacterial activities examined through agar well diffusion method both strains were incubated L-shaped glass rod. Sample dissolved in (DMSO) due to no zone of inhibition and Ciprofloxacin (5 μg/50 mL) was taken as standard drug (Positive control) purchased from Himedia, Mumbai, India. Concentrations was taken as the dose-dependent activity sterile micropipette tips used for the appropriate amount of sample, control [24] and standard and plate were incubated left over at 37˚C for 36 hrs after time duration the antibacterial activity result showed that Compound (8) is active at high concentration 200 to 400 μg/mL [25] .
2.14. Analgesic Activity
Swiss strain albino mice either sex weighing 25 - 30 g were used for this study [21] . The test compounds (in 1/10 the dose of the average LD 50 values of titled compounds) were injected intraperitoneally 10% v/v.
3. Results
The results obtained from the synthesized compounds with a dose of 100 mg/kg confirmed that maximum activity was obtained when X was substituted by halogen (Compound-8) with 74.73% inhibition, when X was substituted by a chlorine group (Compound-2) with 72.90% inhibition; X was substituted by -NO2 group (Compound-3) with 70.80% inhibition, X was substituted by -N (CH3)2 group (Compound-5) with 32.85% inhibition, X was substituted by -OH group (Compound-4) with 49.27% inhibition, X was substituted by -C6H5 group (Compound-1) with 36.86% inhibition. Based on the “p” value, Compound-2 and 3 showed higher significance from 1 hr to 5 hrs when compared with control [26] . It was found that the electron withdrawing groups and alkene containing synthesized compounds enhanced the anti-inflammatory activity. The effect of diclofenac sodium and test compounds on paw thickness shown in Figure 4 and percentage inhibition of paw thickness shown in Figure 3. The percentage
![]()
Figure 3. Bar diagram with mean and standard error of mean at 1 Hr - 5 Hr.
![]()
Figure 4. Effect of Diclofenac sodium and test compounds on paw thickness.
of inhibition was calculated and they were compared with positive control drug. The results showed that the Compound-2 & 3 and other series were active in the assay system used.
4. Applications
The antibacterial activity, of the synthesized 1,2-diazole derivatives were effective against gram positive and gram negative organisms respectively. The antifungal activity, of the synthesized 1,2-diazole derivatives showed good activity against tested fungi. The present study revealed that, synthesized compound N1-(4-hydroxy benzoyl)-3-methyl-5-phenyl-4(N-4-chlorophenylazo)-1,2-diazole possess significant diuretic activity at 100 and 200 mg∙kg−1 but the effect declined at higher dose.
5. Conclusions
The synthesized novel 1,2-diazole derivatives were subjected to in vivo anti-inflammatory evaluation. Anti-inflammatory activity of the synthesized compounds was evaluated by induced diclofenac sodium rat paw edema method. The activity was studied at the dose levels of 100 mg/kg body weight, and their effects were measured at 1 hrs, 2 hrs, 3 hrs and 5 hrs.
The paw volume of the rat in inhibiting inflammation by the synthesized compounds at different time intervals is measured by mercury displacement method. The anti-inflammatory studies revealed that all the synthesized novel 1,2-diazole derivatives showed significant anti-inflammatory activity, when compared with that of standard drug diclofenac sodium. NP-2 and NP-3 showed greater pharmacological activity due to the presence of -Cl and -NO2 and electron withdrawing groups [26] , whereas, NP-5, NP-4 and NP-1 showed mild to moderate activity [27] [28] .