Effect of Light Intensity on Leaf Photosynthetic Characteristics and Accumulation of Flavonoids in Lithocarpus litseifolius (Hance) Chun. (Fagaceae) ()
1. Introduction
Environmental changes can lead to changes of shape, surface characteristics, curl degree and anatomical characteristics of leaves. Light is one of the most important environmental factors affecting the growth and development of plants ( Pengelly et al., 2010 ; Jiang et al., 2011 ). Light not only influences the morphology of a single leaf, but also the overall plant morphology. The plant can adapt to the light intensity changes by changing biomass distribution and its own morphology, so as to maximally utilize the light energy and survive (Xue, 2001; Hu et al., 2006) .
Changes in light intensity can influence leaf photosynthesis. Under weak light, the contents of photosynthetic pigments, especially chlorophyll (Chl) b, will increase. Simultaneously, the chlorophyll a/b ratio (Chl a/b) decreases, accompanied by a decline in maximum net photosynthetic rate (Liu et al., 2012; Wang et al., 2012) . Under strong light conditions, the excess photons absorbed by pigments will not be completely consumed in photosynthesis. As a result, photoinhibition occurs (Shirke & Pathre, 2003) , leading to the generation of a large amount of reactive oxygen species (ROS) and hence oxidative stress. The antioxidant enzymes in plants can scavenge the superoxide anion radicals (Maruta et al., 2010) . Studies have shown that light intensity has a significant impact on the contents of such secondary metabolites as alkaloids ( Zhao et al., 2001 ; Dai et al., 2004) , sesquiterpenes (Hägele et al., 1999) , salidroside (Yan et al., 2003, 2004) , flavonoid glycosides and terpene lactone (Leng et al., 2002; Gavin & Durako, 2012) .
Lithocarpus litseifolius (Hance) Chun., family Fagaceae, is also known as sweet tea. As an evergreen plant, L. litseifolius can simultaneously be consumed as a drug and a food (He et al., 2012a, 2012b) . It is primarily found in mountainous regions of Jiangxi, Guangxi and Hunan, China, as a common evergreen tree species that is heliophilic and can resist drought (Chen & Huang, 1998) . The tender leaves of L. litseifolius are used as traditional Chinese medicine with the functions of clearing heat, promoting diuresis and preventing ulcers, sores and damp-heat dysentery (Xie, 1987) . L. litseifolius has been used for more than 1000 years, exhibiting unique efficacy in lowering blood glucose and lipid levels and blood pressure (Hou et al., 2011; Dong et al., 2012) . The main active components are flavonoids (Li et al., 2008) . Existing studies on L. litseifolius have mostly concerned the extraction and isolation of flavonoids from leaves (Li et al., 2008, 2010) and their applications (Ren et al., 2012) . To avoid wild natural resources have been exhausted, it is necessary to carry out the standardized cultivation. The present work examined the physiological responses of L. litseifolius under different light intensity, in order to make clear the variation of leaf photosynthetic physiological characteristics and accumulation of flavonoids rule. So we can assessment the influence of different light intensity to growth of L. litseifolius and the accumulation of flavonoids in leaves. Thus, appropriate cultivation conditions were determined for increasing the content of flavonoids in L. litseifolius.
2. Materials and Methods
2.1. Experimental Site
The experiment was carried out in the Medicinal Herb Garden of the West Campus, Huaihua University, Hunan Province, China (27˚34'38.78''N, 110˚01'19.44''E). The annual average temperature in this region is 16.4˚C. The precipitation is ample, with annual precipitation of 1027.6 - 1701.3 mm, and annual sunshine duration is 1303.5 - 1519.2 h. This region has a humid mid-subtropical monsoon climate.
2.2. Experimental Materials
The experiment was carried out in 2010, using 3-year-old L. litseifolius with uniform growth status as the experimental material. The shading treatment was applied with black shading net from 15 March 2010 to 15 March 2011, which was installed at a height of 2.5 m above the ground. The light intensity was adjusted by changing the number of shading nets, and the value of shading rate was accurately measured with an illuminometer. There were two light intensity treatments: about 60% and 20% of natural light, using one or two shading nets, respectively. The control was natural light treatment (i.e. no shading net). Conventional cultivation management was implemented. Six plants were cultivated in each plot, with plant and row spacing of 1.5 m. To avoid mutual shading, the spacing between plots was 1.5 m. There were a total of nine plots, with three replicates per treatment.
2.3. Determination of Chlorophyll Content
Following the method of Arnon (1949) , the fresh leaves 0.2 g, which 5 days before treatment and after treatment, respectively. Pigments were extracted with 95% ethanol, then constanted volume to 25 ml. Absorbance values were measured with 7230G spectrophotometer at 665 nm, 649 nm and 470 nm, respectively. Each sample was measured three times and the mean was taken.
2.4. Determination of Net Photosynthetic Rate
A LI-6400XT Portable Photosynthesis System was used in open gas path mode. The leaves of L. litseifolius (three plants per treatment, four leaves per plant, three measurements per leaf and a total of 36 replicates), which fully developed and similar position, that were fully illuminated were randomly selected from the same leaf position under different shading rates, The net photosynthetic rate (Pn) was measured every 2 h during 08:00-18:00 in sunny weather (26-31 August) and the daily variation trend of photosynthetic rate was obtained. The net photosynthetic rate was measured at different photon flux densities (PFDs, 0 - 2000 μmol・m?2・s?1). The PPFD curve was fitted using the method of Bassman and Zwier (1991) .
 (1)
(1)
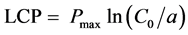 (2)
(2)
 (3)
(3)
Pmax is the maximum net photosynthetic rate, a is a weak light quantum efficiency, that is, apparent quantum efficiency, C0 is a measure of the net photosynthetic rate of the weak light tend to be 0. LCP is light compensation point, LSP is light saturation point.
2.5. Determination of Antioxidant Enzyme System and Soluble Proteins
Superoxide dismutase (SOD) activity was measured using the method of Giannopolitis and Ries (1977) . 0.5 g of vein-free leaves were mixed with 2 mL of 0.1 M phosphate buffer and ground on ice bath. Supernatant was obtained by centrifugation at 10,000×g for 30 min, diluted to 10 mL as crude enzyme and stored at 4˚C for future use. 0.05 mL of the crude enzyme was further diluted to 3.0 mL with water containing 0.3 mL of each 130 mM methionine, 0.75 mM nitrogen blue tetrazolium (NBT), 0.1 mM EDTA-Na2 and 0.02 mM riboflavin and 1.5 mL 0.05 mM phosphate buffer. Enzyme was replaced with PBS buffer in control tubes. One control tube was set in the dark, the other control and the testing tubes were illuminated with 4000 lx fluorescent light for 20 min. Absorptions at 560 nm were measured by referencing to the absorption of the unilluminated control tube. Total and specific SOD activities were calculated based on the following equations: total SOD activity = (ACK ? AE) × V/(0.5 × ACK × W × Vt), specific SOD activity = total SOD activity/protein content, where ACK was the absorption of illuminated control; AE was the absorption of tested samples; V was the total sample volume [ml]; Vt was the tested sample volume [ml]; W was fresh sample mass (FM) [g]; the unit of protein concentration was mg∙g−1 (FM).
Peroxidase (POD) activity was measured by guaiacol method (Chance & Maehly, 1955) . 0.3 g leaves were mixed with 6 mL of 0.1 M phosphate buffer (pH 7.0) and grind it to homogenate. The solution was centrifuged for 30 min at 12,000 g, 4˚C. The clear supernatant was enzyme and stored at 4˚C for future use. The reaction mixture was 50 mL 0.1 M phosphate buffer, 28 μL guaiacol, and 19 μL H2O2 (30% w/v). The absorbance was measured at 470 nm for 3 mL enzyme and 3 mL reaction mixture, respectively. POD activity = ΔA470 × VT/(W × VS × 0.01 × t), △A470 is changes of the absorbance in the reaction time; VT is total extraction enzyme volume; W is the sample weight; VS is the volume of enzyme solution for the determination; t is reaction time.
The malondialdehyde (MDA) content was measured by thiobarbituric acid chromatometry (Buege & Aust, 1978) , 0.3 g leaves were mixed with 2 mL of 0.05 M phosphate buffer and 5 mL 0.5% (W/V) thiobarbituric acid at 100˚C water bath, then centrifuged for 10 min at 4000 g. The clear supernatant was collected and stored at 4˚C for measure. The absorbance was measured at 600 nm, 532 nm and 450 nm. MDA content = [6.452 × (D532 − D600) − 0.559 × D450] × VT/VS × W; VT is total extraction volume; VS is the volume of solution for the determination; W is the sample weight.
Soluble protein content by the method of Bradford (1976) . 100.0 g leaves ground into a homogenate, then centrifuged for 10 min at 4000 g, 4˚C. The clear supernatant was mixed with distilled water to 10 mL as the protein content to be tested. The standard curve was drawn with BSA-standard solution. The absorbance was measured at 595 nm. Soluble protein content = protein quality × VT/VS × W; Protein quality data were obtained according to the standard curve; VT is total extraction volume; VS is the volume of solution for the determination; W is the sample weight.
2.6. Determination of Flavone Content
Flavone content was measured according to the method of Li et al. (2008) . Of leaf samples, 5 g was weighed, pulverized and ultrasonically extracted. After evaporation, the ethanol was recovered, and the solution was diluted to 100 mL with water, then 1 mL of the sample solution was transferred to a 25-mL colorimetric tube. After the addition of 1.0 mL of 5% sodium nitrite, the solution was shaken well and stood for 6 min. Then 1.0 mL of 5% aluminum chloride was added and shaken well, and the tube stood for 6 min. Then 10 mL of 1 mol/L sodium hydroxide was added, and the water was added to 25 mL. The solution was shaken well and stood for 15 min. The absorbance was measured at 510 nm. Total flavones were calculated using a regression equation.
2.7. Statistical Analyses
SPSS13.0 (SPSS) was used for the statistical analysis and normality testing of the data. Single-factor analysis of variance and the multiple comparison of least significant difference (LSD) method were employed.
3. Results and Analysis
3.1. Influence of Light Intensity on the Content of Photosynthetic Pigments
Chlorophyll traps the light energy used for primary reactions of photosynthesis, and plays an important role in light energy transfer and conversion. Chl a, Chl b and Chl (a + b) all increased with higher shading rates (Figure 1); however, the Chl a/b ratio decreased. This indicated that under weak light conditions, the leaves mainly absorbed the blue-violet and orange light in the diffused light. As the shading rate increased, Chl (a + b) of leaves increased. As Chl b increased, the leaves of L. litseifolius could absorb the scattered light and thus maintain a high photosynthetic rate.
3.2. Influence of Light Intensity on Photosynthesis
Light intensity is one of the major environmental factors influencing plant photosynthesis. Pn decreased as shading rate increased (Figure 2). Under full light conditions, the Pn of L. litseifolius varied as double-peaked curve, indicating inhibition of photo-
![]()
Figure 1. Chlorophyll contents in L. litseifolius leaves under different light treatments. Different letters indicate significant differences under different light treatments plants (t-test, p < 0.05).
![]()
Figure 2. Diurnal variation of photosynthesis rate (Pn) under different light treatments in L. litseifolius leaves.
synthesis at noon. With increased shading intensity, Pn decreased. The daily average Pn was 7.71, 6.27 and 5.23 μmol CO2 m−2・s−1 under natural light and 60% and 20% natural light, respectively, with significant (p < 0.05) differences between the treatments.
3.3. Photosynthetic Rate-Light Intensity Curve
Light compensation and saturation points are direct indicators of the capacity of plants to utilize light. They can be used to evaluate the shade tolerance of plants. The light response curve of L. litseifolius under different shading rates showed some differences (Figure 3). The results calculated from the P?PFD curve and the corresponding equations are shown in Table 1. The light compensation points under full, 60% and 20% natural light occurred at 31, 10 and 19 μmol・m?2・s?1, respectively. The saturation points were at 1528, 1167 and 1038 μmol・m?2・s?1, corresponding to maximum net photosynthetic rates of 7.8, 6.1 and 5.10 μmol・m?2・s?1. Shading caused declines in light compensation point, saturation point and maximum Pn to varying extents. The apparent quantum efficiency (AQY) was 0.031, 0.042 and 0.027, respectively, i.e. 60% natural light > full light condition > 20% natural light. A higher AQY indicates an accelerated photosynthetic rate at higher light intensity and a greater sensitivity to light intensity. Of the three treatments, the leaves of L. litseifolius were most sensitive to weak light under 60% natural light.
3.4. Influence of Light Intensity on Antioxidant Enzyme System and Soluble Proteins
Shading caused significant declines in SOD and POD activities, as well as a non-signif- icant decline in content of soluble proteins (Table 2). Compared to full light, SOD activity decreased by 7.69% and 55.78% as the shading rate increased, respectively; and correspondingly POD activity decreased by 13.66% and 38.86%. The content of soluble proteins did not decrease significantly, going from 5.48 mg at full light condition to 5.25 and 4.68 mg at 60% and 20% natural light, or declining by 3.33% and 14.8%, respectively. MDA content showed an increasing trend with increased shading rate, i.e. by 7.93% and 33.3%, respectively, compared to full light.
![]()
Table 1. Light response characteristics in L. litseifolius leaves under different light treatments (means ± SE).
![]()
Table 2. Antioxidant enzyme system in L. litseifolius leaves under different light treatments (means ± SE). Different letters indicate significant differences under different light treatments plants (t-test, p<0.05 and p < 0.01).
Note: Different uppercase and lowercase letters in the same column indicate significant differences of parameters under different light treatments at 0.05 and 0.01 levels, respectively.
![]()
Figure 3. The photosynthetic light response curves of L. litseifolius under different light treatments.
3.5. Influence of Light Intensity on Accumulation of Flavones
Shading affected flavone concentration in leaves (Figure 4). The flavone content of leaves was highest and lowest at 60% and 20% natural light, respectively. This indicated that appropriate shading was favorable for accumulation of total flavones in leaves of L. litseifolius. As the light intensity decreased, the accumulation of total flavones was inhibited. As the time of shading treatment was prolonged, the flavone content first increased and then decreased. Total flavones were determined after 60 d of shading treatment in the different groups. The total flavone was highest (12.25%) in leaves under 60% natural light, and lowest under 20% natural light, which was only 7.64%. The total flavone content under full light conditions was intermediate, with 10.25%.
4. Discussion
4.1. Influence of Light Intensity on Leaf Photosynthesis of L. litseifolius
The light compensation point is a direct indicator of the ability to utilize weak light. The lower the light compensation point, the easier photosynthesis will be under weak light conditions. Light intensity exceeds the light compensation point, as light intensity increases, photosynthetic rate is gradually increased, and photosynthetic rate is more than respiration intensity, which promotes the accumulation of organic matter in plants. A lower light saturation point usually indicates that the photosynthetic rate rapidly reaches a maximum as photosynthetically active radiation increases. Shading caused a decline in light compensation and saturation points of leaves of L. litseifolius, shows that L. litseifolius could fully utilize weak light for photosynthesis. Apparent quantum yield (AQY) reflects the photosynthetic capacity of leaves under weak light conditions. A higher AQY indicates a greater amount of light-harvesting complex for
![]()
Figure 4. The content of total flavonoids in L. litseifolius under different light treatments.
light energy absorption and conversion and hence a greater ability to utilize weak light (Richardson & Berlyn, 2002) . AQY was highest for the 40% shading rate, suggesting that L. litseifolius could increase the photosynthetic rate through internal regulatory mechanisms in response to reduced light intensity.
Chlorophyll content and Chl a/b ratio are usually used to distinguish between heliophytes and shade plants. For shade plants, the chlorophyll content per unit leaf area is higher, but Chl a/b ratio is lower (Murchie & Horton, 1998; Baig et al., 2005) . The chlorophyll content in L. litseifolius was significantly lower under full light than under shaded conditions; however, the Chl a/b ratio was obviously higher than that under shaded conditions. The changes of leaf photosynthetic rate in the present work showed that L. litseifolius was a heliophyte and was resistant to drought. Under shaded conditions, Chl (a + b), Chl a and Chl b contents all increased, while the Chl a/b ratio decreased. The increase of chlorophyll content can promote photosynthetic capacity (Bailey et al., 2001) . Under shaded conditions, the light saturation point of L. litseifolius is decreased, and the net photosynthetic rate is still the highest (Figure 2). Chlorophyll b is synthesized at a faster rate under weak light conditions, and a relative increase of Chl b can improve the plant’s capacity to utilize red and infrared light. An increase of light-harvesting ability is a form of adaptation of plants to weak light conditions (Bertamini et al., 2006) . Thus a decrease in Chl a/b ratio shows enhanced light absorption capacity under weak light conditions.
4.2. Influence of Light Intensity on Antioxidant Enzyme System
The balance of reactive oxygen species (ROS) metabolism is disrupted in plants under stress?the activity of ROS scavengers such as SOD and POD decreases and the ROS content increases (Wang et al., 1989; Maruta et al., 2010) . The increase of ROS causes membrane lipid peroxidation, leading to damage to cell membranes. As a product of membrane lipid peroxidation, MDA can be used to characterize the degree of this reaction and the plant’s response to stress (Zhu et al., 2009) . For heliophytes, the light energy utilization rate is 25% under strong light. However, for shade plants, light saturation is reached at 5% of strong light, showing a lower light energy utilization rate (Long et al., 1994) . Excess light energy causes photooxidation of chloroplasts, which in turn generate ROS that damage the photosynthetic organism (Long et al., 1994; Shirke & Pathre, 2003) . The SOD activity of L. litseifolius was higher under full light than under shaded conditions. Thus, although the photosynthetic capacity was improved compared with shaded conditions, the excess energy light caused the photooxidation of chloroplasts and generation of ROS. Under weak light conditions, the leaves of L. litseifolius can efficiently utilize light energy for photosynthesis, which reduces the plant damage caused by ROS. Due to low SOD activity, a large amount of ROS will accumulate, damaging the chloroplast envelope and increasing the MDA content. Shading decreased the POD activity of L. litseifolius, this indicated that under weak-light stress, the dynamic balance between the generation and decomposition of free oxygen radicals was disrupted. The yield of ROS exceeded the scavenging ability of the antioxidant enzyme system, leading to membrane lipid peroxidation and cell damage.
Proteins are the important structural and functional substances in plants, the metabolism of which is regulated by various factors. Increasing evidence shows that a changing environmental factor or stress will influence protein metabolism (Longstreth et al., 1980) . The soluble proteins in plants are mostly enzymes involved in metabolic processes. A weakening of light intensity can influence the normal physiological and biochemical processes of plants, leading to the disordered metabolism of proteins. The activity of protective enzymes and the soluble protein content may be reduced under stress, thereby increasing the generation of ROS which damage the cell membrane. With a significant decline in antioxidant ability, the plants will experience rapid senescence. Although L. litseifolius could adapt to weak light conditions by reducing leaf thickness, its tolerance of weak light was limited due to the reduced activity of SOD and POD and the resulting increase of ROS.
4.3. Influence of Light Intensity on Flavonoid Accumulation
Flavonoids, which are secondary metabolites, have a protective effect against ultraviolet radiation in plants. As light intensity increases, the intensity of ultraviolet radiation increases accordingly. The plant requires more accumulation of total flavones for stronger protection (Su et al., 2006) . In Ginkgo biloba, shading can significantly decrease the content of flavonoid glycosides (Leng et al., 2002) . Shading treatment can also reduce total flavone content in Erigeron breviscapus (Su et al., 2006) . However, the flavone content in L. litseifolius was not linearly related to light intensity. The flavone content was highest in leaves at 40% shading rate, and was lower under full light than 80% shading. It can be inferred that there is an optimal light intensity for accumulation of flavones in L. litseifolius, below or above which the flavone content will decrease.
The variation of flavonoid accumulation differs between plant species, which may be related to the complex metabolic pathway of flavonoids. The biosynthesis of flavonoids starts from the phenylpropanoid pathway. Other synthetic pathways are regulated by L-phenylalanine ammonia lyase (PAL), chalcone synthase (CHS), chalcone isomerase (CHI), dihydroflavonol 4-reductase (DFR) and isoflavone synthase (IFS) (Brenda, 2001) . In Fagopyrum esculentum, total flavone varies in the same direction as PAL activity (Tang & Zhao, 1992) . Enhanced photosynthesis provides more precursors of secondary metabolites and inhibits the decomposition of secondary metabolites. Therefore, a higher photosynthetic capacity can increase the flavone content in leaves. As an inducible enzyme, PAL can be induced by various factors. In addition to flavonoids, the products of the phenylpropanoid pathway also include lignin and alkaloids (Ouyang & Xue, 1988) . Hence, the flavone accumulation caused by shading may show different features between plant species (Zhao et al., 1999) . Although L. litseifolius had its highest photosynthetic capacity under full light conditions, the flavonoid content was not the highest. This may be because it is a heliophyte, and is more easily adapted to full light conditions. Under lower light intensity, the activity of antioxidant enzymes in L. litseifolius decreased, causing increases in ROS. More flavonoids were synthesized to scavenge the ROS, in order to protect the plant.
The content of flavonoids in L. litseifolius leaves was correlated with the time of shading treatment (Figure 4). As the time of shading treatment was prolonged, the content of flavonoids gradually increased. After 60 d of shading, the content of flavonoids in the leaves began to decrease. A possible reason for this is that the weak-light stress over the long-term caused senescence of cells and the decline of synthetic ability.
4.4. Selection of Cultivation Sites of L. litseifolius
Although there will be a decrease of dry weight of a single leaf due to the weakened photosynthetic capacity under weak light, the biomass distribution to leaves will increase (Tao & Zhong, 2003; Wang & Wei, 2010) . Under 20% shading rate, the biomass in leaves of entire plants of Catharanthus roseus did not differ significantly from that under full light condition. However, the contents of vindoline and catharanthine increased significantly (Tong et al., 2011) . Thus the flavone content was the highest in the leaves of L. litseifolius under 40% shading rate, and therefore the flavone yield was the highest.
The sun is located more to the south in non-tropical regions in the Northern Hemisphere. Therefore, the South-facing slope receives more light energy than flat ground, which results in a higher total solar radiation and temperature than for the North-facing slope. However, humidity is obviously lower for the South-facing compared to the North-facing slope. For this reason, the South-facing slope is usually called the “sunny slope”, and the North-facing the “shady slope”. The environmental conditions of East-facing and West-facing slopes lie between those of the South-facing and North-facing slopes. The West-facing slope is illuminated by solar radiation for longer hours in a day than the East-facing slope, and so its environmental conditions are closer to those of the South-facing slope. The West-facing slope is called the “semi-sunny slope”, and the East-facing slope the “semi-shady slope” (Yin, 2004) . The plant communities in South-facing slopes are type of the South flora, showing feature of require less water and greater heat, while plant communities in North-facing slopes can resist cold as the North flora (Yan et al., 2011) . As to the aspect, the upper slope receives ample solar radiation and obtains enough heat, which benefits the growth of heliophytes. The lower slope is dominated by high-adaptability or shade-tolerant species (Ma et al., 2010) . Lithocarpus litseifolius requires light and also has some resistance to shade. As appropriate shading will induce accumulation of flavonoids, L. litseifolius should be cultivated at lower positions of sunny slopes or upper positions of shady slopes. It could also be cultivated in West-facing and East-facing slopes.
5. Conclusion
Lithocarpus litseifolius (Hance) Chun. has high net photosynthetic rate. These features enable L. litseifolius to survive in habitats with sufficient sunlight. After appropriate shading, the leaves of total chlorophyll content increased, while the Chl a/b ratio decreased, as a response to weakened light intensity. Under weak-light stress, SOD and POD had lower activity and the accumulation of free radicals caused cell damage and the rise of intracellular MDA content. In the meantime, the content of soluble proteins declined. Under weak-light stress, L. litseifolius accumulated flavonoids to resist this stress. As the leaves grew, the flavone content of leaves declined. Therefore, L. litseifolius should be cultivated in lower positions of sunny slopes or upper positions of shady slopes to promote accumulation of flavones in leaves.
Acknowledgements
This work was supported by the Construct Program of the Key Discipline in the Education Department of Hunan Province (201142), Science and Technology Plan Project of Hunan Province (2014NK3109).

*These authors contributed equally to this study.