Long-Term Results of Balloon Angioplasty for Native Coarctation of the Aorta in the Surgical Specialty Teaching Hospital/Cardiac Center/Hawler ()
1. Introduction
Coarcation of Aorta (COA) occurs in 8% to 10% of all cases of congenital heart defect. It is more common in male than female (male/female ratio of 2:1). Among patients with Turners syndrome, 30% have COA with a higher incidence in stillbirth infants [1] - [4] .
Constrictions of the aorta of varying degrees may occur at any point from the transverse arch to the iliac bifurcation [1] . The common form of COA of aorta is represented by a localized constriction containing the ridge or shelf, more common than tubular hypoplasia [5] . The abdominal aorta is rarely involved [4] . COA of the abdominal aorta can be part of a systemic vascular disorder such as Takayasu arteritis or von Recklinghausen disease but also can be congenital [6] .
COA occurs as of other congenital heart defects such as bicuspid aortic valve (as many as 85% of patients with COA have bicuspid aortic valve), transposition of great arteries, double outlet right ventricle (taussing being anomaly), discordant ventriculo- arterial connection, common arterial trunk, ventricular septal defect (defects are more frequently perimembranous and associated with postero-inferior overriding of the aortic valve), interruption of the aortic arch which can be found with an aortopulmonary window rather than VSD, and mitral valve abnormalities (a supravalvular mitral ring or parachute mitral valve, and subaortic stenosis are potential associated lesion, when this group of left-sided obstructive lesions occurs together, they are referred to as the shone complex) [1] - [3] [7] . Bicuspid aortic valve disease is a big contributor to cardiac failure, which in turn makes up roughly 20% of late deaths to coarctation patients [7] . The diagnosis of COA in older children and adult usually follows a routine medical examination, where the murmur is discovered; weak femoral pulse, or unexplained systemic hypertension is found; headache, nosebleeds, cold feet or calf pain on exercise is often experienced. The major complications of COA are congestive heart failure, subarachnoid hemorrhage, infective endocarditis and aortic dissection; dissection is particularly dangerous in pregnant women [5] [8] .
The pathogensis of systemic hypertension in COA of aorta has been the subject to the theory that the mechanics focuses on resistance at the site of obstruction. This theory in itself cannot be sustained, but it is central to the neural theory that involves the distensibility characteristics of the pre COA aorta, the sensitivity of carotid sinus baroreceptors and relatively non compliant precoarctation aorta [9] [10] . Also elastic properties of the normal aorta decrease with distance from the aortic root. In COA of aorta, the proximal aortic segment is characterized by an increase in collagen and a decrease in smooth muscle [11] .
There are two pathognomonic signs on the plain CXR in older children. The first is figure-3 sign. The upper arc of the 3 is the dilated arch proximal to the COA and/or a dilated left subclavian artery. The lower arc or bulge represents post stenotic dilatation of the aorta immediately below the COA. The indentation between the 2 bulges is the COA itself. When the esophagus is filled with barium, a reverse 3 or E sign is often seen; it is a mirror image of the areas of prestenotic and poststenotic dilatation [1] [12] . The second sign is rib notching, which is usually not seen until 4 years of age. It is best seen posteriorly in the medial third of the lower borders of the fourth to eight ribs, where the intercostals artery cross the rib notching is present in 75% of adults with COA. It is caused by pulsation of dilated intercostals arteries [1] [13] [14] . MRI vividly portrays the anatomy of the COA. It also demonstrates the bicuspid valve and the state of left ventricular function, as well as restenosis following angioplasty or surgical repair. The degree of confidence is very high [1] [15] - [18] .
Echocardiography: There is most commonly a short narrowed segment just distal to the left subclavian artery caused by the obstructive shelf projecting into the aorta posteriorly. More rarely there is a longer segment of narrowing involving the isthmus [1] [2] [19] - [21] . Doppler examination often demonstrates a pattern of diastolic runoff, especially in patients with robust collaterals or tight stenosis [2] . However, in the presence of a patent ductus arteriosus, the severity of the narrowing may be underestimated [22] .
Cardiac Catheterization and Angiography: one potential indication is for inter- venesion in the setting of COA. Controversy has surrounded the use of balloon angioplasty as the primary treatment for patients with various ages. The first operations to treat COA were carried out by Clarence Crafoord in Sweden in 1944 [1] [23] [24] .
Angioplasty is a procedure done to dilate an abnormally narrow section of a blood vessel to allow better blood flow, After a COA repair 20% - 60% of infant patients may experience reoccurring stenosis at the site of the original operation [25] . Immediate and intermediate-term follow up are generally good with a small chance for recoarctation and aneurismal formation at the site of COA. The causes of recoarctation were identified and include age less than 1 year, isthmus hypoplasia, and a small coarcted aortic segment. [26] [27] and long-term follow up studies are required prior to recommending angioplasty as an alternative to surgical therapy in adult patients [28] .
American Heart Association released in 2011. Angioplasty for native COA is a Class IIa indication as a palliative measure to stabilize a patient with severely depressed ventricle function. In addition, Class IIb indications include patients beyond four to six months of age with suitable anatomy (discrete COA). Regarding stenting, Class IIa indications include use of stents that can be dilated to adult size diameters in native (discrete or long segment) COA when angioplasty has failed [29] .
A number of potential factors may be responsible for the development of aneurysms after percutaneous balloon angioplasty, Previous histologic studies demonstrated extensive intimal and medial tears in resected COA segments that were dilated. Cystic medionecrosis, by contributing to the progression of intimal and medial tears, could lead to the adverse effects now being reported after balloon angioplasty [30] .
Another probable mechanism of aneurysm formation after angioplasty is over distension may cause complete a complete trans medial tear resulting in loss and disarray of supporting smooth muscle cells causing weakening in the vessel wall, Anurysm following dilation occur in 2% - 5% of patients and appears related to degree of anatomical enlargement [31] .
2. Patients and Methods
Study design: case series (case follow up study).
Between March 2008 and February 2015, percutaneous balloon angioplasty was attempted in 110 patients with native COA of aorta for duration of more than one year in both gender between age 1 - 21 years of age who had been admitted to surgical specialty hospital/cardiac center which the only center in Erbil which deals with cardiac problems. A total of 50 patients were included in our study, 31 (62%) male and 19 (38%) female, the remaining other 60 cases were lost for follow up because they did not keep any contact with hospital.
Patients who underwent stent implantation or surgery for repair of COA, those with balloon angioplasty for less than 1 year and those with age more than 21 years, are excluded from the study. Data for 50 patients by recall them, were obtained by direct interview and examination of the patients asking for their consent for full ethical consideration, and from hospital based medical records. Case notes of all patients meeting including clinical examination, body weight, height, Blood pressure measurements, radiographic, Echo Doppler data, CT angiography were obtained, the mean duration of follow up visit for long term complications after the procedure was 3.02 ± 1.59 years. Measure blood pressure in the supine position with standard sphygmomanometric auscultatory method without discontinuation of antihypertensive medication. Measured Systolic and diastolic blood pressure were plotted on auscultatory BP percentiles curves according to age for boys and girls. Echo-Doppler examinations (VIVID 3 generation machine) were performed on follow up visit. A two, three or five MHz probe was used for examination. The maximum peak instantaneous gradient across the previously dilated coarctated area was determined by using a standard Doppler approach and calculated using the modified Bernoulli equation [2] . The spectral recording shows an extension of antegrade flow, and persisting gradient into diastole, so called diastolic tail. The spectral recording can be analyzed further according to peak velocity and the half-time of diastolic velocity decay. This predicts accurately severity of anatomical obstruction [1] . Full catheterization data were reviewed including the systolic, and mean pressure gradient of ascending and descending aorta (peak-to-peak systolic gradient) before and after balloon angioplasty.
Statistical analysis: Data were analyzed using the Statistical Package for Social Science (SPSS version 19). Mean and standard deviation used for numerical data, Paired T test was used to compare mean between readings before and after the procedure, P value of ≤0.05 was considered as statistically significant.
Limitation of the Study: one of the limitation of the study that we did a several trails to contact with cases, but they did not response to us, may be because of they are far away from the center or economic factors and security factors because of many patients leave in the middle and south Iraq which is difficult to come to north of Iraq (our situation) another limitation was many of patients refuse to do CT angiography [31] .
Results: The current study included 50 patients with congenital native COA in whom balloon angioplasty done for more than 1 year duration at the time of follow up visit to evaluate the complication of balloon angioplasty of both gender between the age of 1 - 21 years old CT angiography done for 34 patients for the evaluation of the complication of balloon angioplasty at the follow up visit, the remaining 16 cases of them refuse the CT angiography, The clinical and demographic characteristics of the studied patients at the time of follow up visit are illustrated in (Table 1), the mean age of patients was 10.37 ± 6.42 years (range 1.3 - 21 years), with a male predominance: male/ female = 31 (62.0%/19 (38%), mean duration of follow up evaluation visit after balloon angioplasty was (3.02 ± 1.59) years, range between (1 - 6.5 years) (Table 1).
Systolic blood pressure measurements in the supine position with standard sphyg- momanometric auscultatory method without discontinuation of antihypertensive medications and data plotted on chart of both gender of Normative BP percentile values. Systolic and diastolic BP mean and Standard deviation are (114.46 ± 23.85), (59.54 ± 17.36) respectively. Chart 1 and Chart 2 show number and percentage of measured systolic and diastolic BP percentile respectively.
![]()
Table 1. The clinical characteristics of patients with COA after Balloon angioplasty.
N = patient number; SD = standard duration.
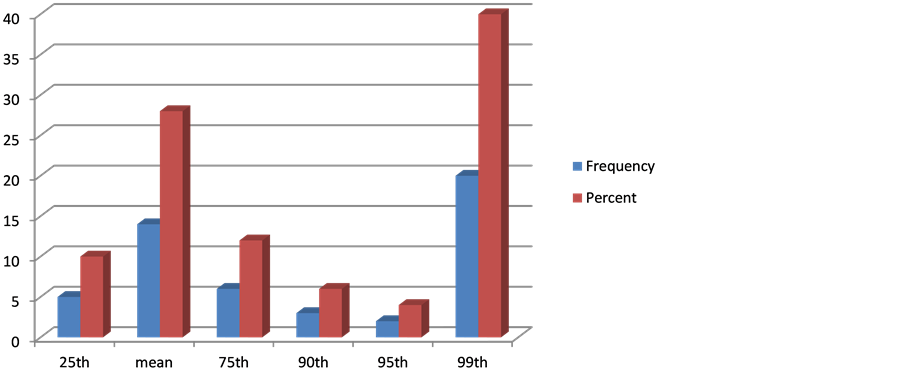
Chart 1. Frequency and percentage of measured systolic BP.
Antihypertensive agents in form of diurectis (fursemide, aldactone), ACI inhibitor (Enalpril, captopril), adrenergic inhibitors (Propranolol, Atenolol, Metoprolol…), it was found that 17 (34%) of patient were on antihypertensive agents while 33 (66%) on no treatments, Chart 3 shows the number and percentage of patients that on antihypertensive agents and Table 2 shows type of antihypertensive agent.
It has been found that 27 (54%) of Patients had normal CXR, 12 (24%) had cardiomegaly, Rib notching is found in 10 (2%), Notching of the ribs is a classic radiologic signs of COA of the aorta caused by collateral flow through dilated, tortuous, pulsatile posterior intercostals arteries (Tables 3-5) [32] .
The associated cardiac anomaly is found among 24 (48%) of patients with COA of aorta inform of subaortic ridge, PDA, AS, MS, mitral valve prolapse…
Turner syndrome is found among 3 patients.
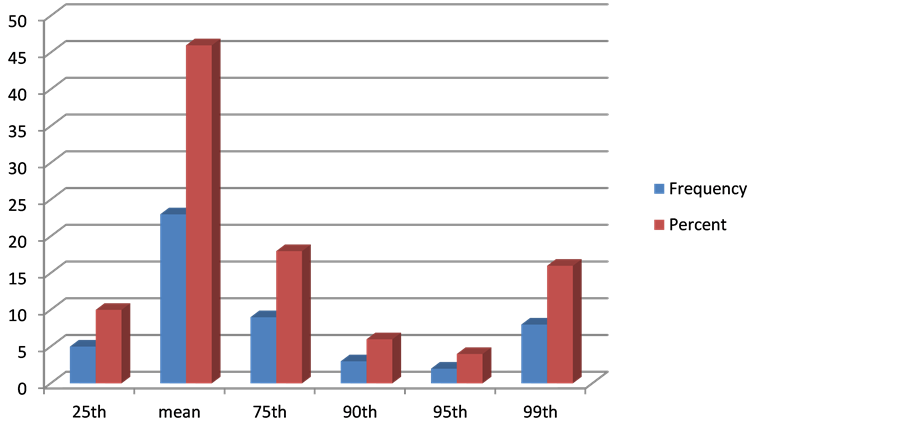
Chart 2. Frequency and percentage of measured diastolic BP.
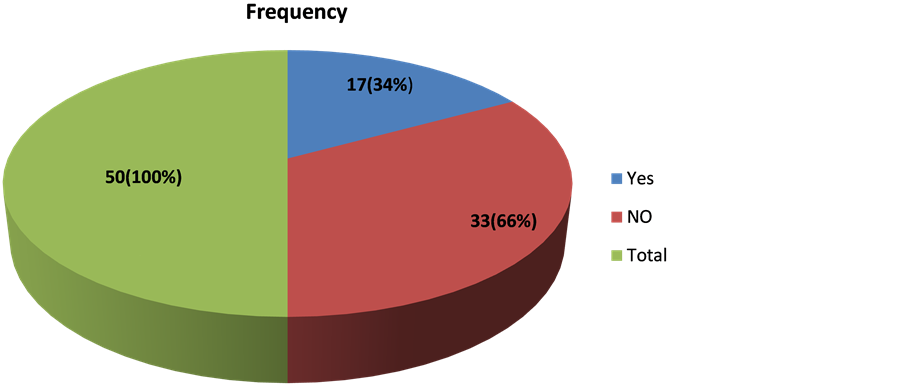
Chart 3. Frequency and percentage of patients with and without antihypertensive agents.
![]()
Table 2. Frequency of the patients who are on antihypertensive agents.
ACI: Angiotensine converting enzyme inhibitor.
![]()
Table 3. CXR finding in patients of coarcation of aorta.
CXR: chest X ray.
![]()
Table 4. No. and type of associated anomaly.
NO: number, PDA: patent ductus arteriosus, AI: aortic incompetence, AS: aortic stenosis, RPA: Rt pulmonary artery stenosis, MS: mitral stenosis, VSD: ventricular septal defect.
![]()
Table 5. Peak instantaneous pressure gradient before and after balloon by Doppler echocardiogram.
SD: standard deviation.
At the follow up visit, it was found that there is significant reduction in the pressure gradient across the coarctated area by Doppler echocardiogram gradient before balloon angioplasty and at the follow up visit from 56.92 ± 15.2 mmHg to 30.6 ± 16.89, P = 0.00.
Paired T test is used to compare mean of peak instantaneous pressure gradient of sample before COA balloon angioplasty and after it, it founded that calculated t test is 9.72 and the tabulated t test (with level of significance is 0.05) is 2.05 so we reject the null hypothesis and we conclude that there is significant difference between the mean of peak instantaneous pressure gradient before and after COA balloon angioplasty.
It was found that one case of which his age between 1 - 4 years with diastolic runoff and significant pressure gradient across the coarctated area by Doppler Echocardiogram referred to surgery, meanwhile three cases of which their aged 5 - 9 years had pattern of diastolic runoff, stent had been put for two of them and the other one referred to surgery, also stent had been put for the one in whom aged 10 - 14 years, the one who older than 15 years missed for the follow up (Table 6).
While the only 6 of total 34 cases who had CT angiography (12%) had the report of CT angiography that documented aneurysm. An aneurysm was defined as an aortic ratio of greater than 1.5, measuring aortic diameter at the coarctated repair site and the thoracic aorta at the level of diaphragm [33] .
3. Discussion
In the present study, we demonstrate a satisfactory long term reduction in the peak instantaneous pressure gradient by Doppler echocardiography from 56.92 ± 14.16 to 30.68 ± 16.89, (P value = 0.00), While 12% (6 of total 34 cases) reported aneurysm formation after follow up evaluation of mean duration (3.02 ± 1.59 years).
A study done in USA who studied 20 patients, which found a decrease in calculated instantaneous COA pressure gradient across COA From mean (43.3 ± 21.9 mmHg) to (to 25.3 ± 10.6 mmHg) for mean duration of follow up of 12 months, (P < 0.01) which is nearly comparable with the our study [33] . The same study reported no aneurysm formation [27] . While 12% in our study, restenosis occur more often with young age and a hypoplastic transverse arch [31] .
Another study which done in Riyadh Saudi Arabia, concluded that the doppler COA gradient across the COA site decreased from 57.6 ± 17.7 mmHg to 16.6 ± 8.4 mmHg
![]()
Table 6. Pattern of diastolic runoff according to the age groups.
one year after angioplasty [34] .
While one done by a group of researchers who studied 26 patients, founded that mean peak systolic blood pressure was (111.1 ± 14.1 mmHg) at long-term follow-up (P < 0.05), which is consistent with our study [35] . In Saudi Arabia found that the measured systolic blood pressure values were (134 ± 18 mmHg), which is incomparable with our study in which measured systolic blood pressure was (114.46 ± 23.85) [36] .
Another study founded that reduction in systolic blood pressure ranged from 167 ± 28 to 132 ± 17 mmHg at the long term follow. While did not demonstrate aneurysm formation in their series [33] .
A prospective case series study done by Munayer Calderon, who studied 333 cases of native COA, reported only 15 people developed aneurysm [37] . While a randomized controlled trial done by Shaddy, in USA done on 36 cases with native COA, age range between 3 to 10 years who founded that 4 case developed aneurysm [38] .
A study done by a group of researchers, founded that three patients from total of 7 cases (43%) reported aneurysm formation [39] .
Another study done in Texas concluded that small aneurysm was found in 2 (1.9%) of 102 patients [40] . In large series of patients 140 patients, they found that 2 early and 6 late aneurysms reported after the COA dilation, which is inconsistent with our study [41] .
Some aneurysms developed late, first being detected more than 5 years after the initial intervention. Only 50% of balloon angioplasty subjects remained free of both aneurysm formation and repeat intervention [26] .
Another researcher who studied 20 children aged 6 - 30 months following dilatation of native COA and 4 children, aged 6 - 22 months following dilatation of recoarctation and none developed aneurysms [42] . The reason for this difference is not clear. It was founded that use of large balloons, inadvertent manipulation of catheter/guide wires in the region of freshly dilated aortic COA, and misinterpretation or over interpretation of aneurysm as possible causes [43] .
In study that done in Texas documented that 88% of patients who had a successful angioplasty maintained a gradient of <20 mmHg over a mean period of 33.8 months follow up [40] .
By follow up angiogram at 12 months and serial MRI up to 10 years after initial dilation were scrutinized for aneurysm formation at the site of balloon angioplasty, three from total of 43 patients were observed to had aneurysm by both an angioplasty and MRI giving the incidence of 7% [44] .
4. Conclusion
Balloon angioplasty of native aortic COA can be performed safely and effectively in most infants, children and adolescents with good immediate and short term outcome. Furthermore, it offers satisfactory long-term results with low incidence of persisting hypertension, recoarctation and aneurysm formation. As long as superior long-term follow-up results of aortic stenting are to be awaited, it thus may be considered as first- line therapy in adolescents and adults with native and discrete COA. Anurysm formation was an unusual complication. Balloon angiography may be considered as the first line therapy in most children with COA.
5. Recommendation
Further long term studies should be undertaken which involve a larger numbers of patients to evaluate results of balloon angioplasty for native COA, and perform angiography to measure peak systolic gradient across the coarctated area more accurately and to know the proportion of cases with recoarctation, Similar to alternatives for angioplasty, life-time surveillance using contemporary imaging techniques is warranted to detect poor outcome in time.
6. Compliance with Ethical Standards
I declare that I have no conflict of interest.
Informed consent was obtained from all individual participants included in the study.