Tin Recovery as Stannous Chloride by Chlorination of Tin-Plated Scrap at 298 K ()

Subject Areas: Analytical Chemistry, Material Experiment
1. Introduction
Tin forms two chlorides, stannic chloride SnCl4 and stannous chloride SnCl2, both of which chlorides are of industrial significance [1] . Stannic chloride is primarily used as the starting material for the preparation of other tin compounds [2] and stannous chloride is the principal component of the solution used for the producing electrolytic tinplate, by the Halogen process [3] which became popular in 1942. Stannous chloride is also a component of the solution used for plating the tin-nickel alloy used in the manufacture of printed circuit boards [4] . Large quantities of stannous chloride are used to stabilize the perfume in toilet soaps. Other applications include use as an additive to drilling muds, tin coating of sensitized paper and use as an anti-sludge agent for oils. Because of its reducing properties, it is widely used in many chemical reactions in both synthetic and analytical chemistry [5] .
Anhydrous stannous chloride can be synthesized by a number of methods. Pure stannous chloride can be made by heating stannous sulphide in a stream of hydrogen chloride. Alternatively, stannous hydroxide can be converted to the chloride [5] by reaction with hydrochloric acid. The most straightforward synthesis technique and the one that is most used are the direct reaction of tin metal and either chloride or tin tetrachloride or the reaction of hydrogen chloride gas with tin. Among the more direct methods, the preparation of stannous chloride by the slow addition of chlorine to molten tin is possible but is difficult to control with regard to the simultaneous formation of stannic chloride [6] . Normal practice [6] consists of bubbling chloride through molten tin at 600˚C - 700˚C and condensing the stannous chloride at 120˚C - 260˚C. In all the synthesis procedures used, the stannous chloride must be condensed between 120˚C and 260˚C [6] [7] - [13] . Although the suggestion of volatilizing stannous chloride is not new, and in that several processes proposed during the last forty years have been based on this approach, it has not yet been possible to achieve economic success. It is, however, possible to prepare stannous chloride by direct reaction of tin from tin plated scrap with chlorine at room temperature. The basic difference in the chlorination temperature and the direct combination of the elements make this process be the simplest one and economically attractive when compared with the above synthesis procedures at high temperature (200˚C to 1080˚C) for SnCl2. Information on the kinetics of reaction of tin from tin plated scrap with chlorine gas at room temperature would be useful for determining the conditions under which SnCl2(s) forms rapidly.
The kinetics of reaction of tin metal with gaseous chlorine has been reported in the literature [14] - [16] , and some information is available on the reaction of tin slags with chlorine [17] - [20] to produce SnCl2. However no systematic studies at low temperatures have been reported in the literature for the chlorination of tin from plated scrap.
From Figure 1, it can be seen that FeCl2(s) is more stable than SnCl2(s) and SnCl4(l). However, SnCl4(l) is highly volatile and apparently, the liquid chloride completely covers the surface of the scrap, so that it is hard to expect that Fe from the tin cans will react with Cl2.
The compound SnCl4(l) has a very low melting point of 265 K and a high vapor pressure of 3.42 KPa at 298 K, as indicated in Table 1. Chlorination to SnCl4(l) can still be achieved with the high vapor pressure of tin chloride acting as the main driving force.
In the present work, detinning of steel scrap was achieved by selective chlorination in nitrogen-chlorine gas mixtures. Thus, by controlling the partial pressure ratio of  [23] - [27] , it is possible to chlorinate selectively the tin from tin plated scrap.
[23] - [27] , it is possible to chlorinate selectively the tin from tin plated scrap.
![]()
Figure 1. Standard Gibbs free energy as a function of temperature for selected iron and tin chlorides.
![]()
Table 1. Thermochemical properties of selected iron and tin chlorides at 298 K [21] .
a. Extrapolated from Barbi et al. data [22] .
2. Experimental
Experiments were carried out with iron scrap (0.5% Sn) supplied by Domingues & Cia (Caracas, Venezuela). The experimental arrangement is shown in Figure 2. The reaction tube (25 mm ID) was made of Pyrex. Commercial nitrogen was passed through two drierite desiccants columns to absorb moisture and through heated BASF copper catalyst at 220˚C and copper turnings at 750˚C to remove traces of oxygen. Chlorine was dried in three bubblers containing sulfuric acid, and was passed through P2O5 to remove water. Mixtures of chlorine and nitrogen were prepared by means of conventional calibrated flowmeters. After passing through the reaction tube, the unreacted chlorine was absorbed in concentrated sodium hydroxide solutions. The iron scrap samples were cleaned with xylene and acetone to remove grease. Before each run the samples were weighed to within ±10−5 grs, and the surface areas used to calculate the reported rates were taken as the geometrical areas of the sample.
To start a run, the samples were inserted in the reaction tube and the system was evacuated by a vacuum pump and back filled with purified nitrogen. After about ten minutes the desired Cl2/N2 gas mixture was passed through the reaction tube for periods of from two to forty minutes. A run was stopped by introducing purified nitrogen until of the liquid chloride product was removed from the surface of the sample. The sample was then removed from the reaction tube, and subjected to study by X-ray diffraction and chemical analysis for tin content.
3. Results and Discussion
Typical variations of the measured weight loss per unit area with time for the chlorination of iron scrap in Cl2/N2 gas mixtures for two gas flow rates (2.0 and 4.0 mil/min) are shown in Figure 3 and Figure 4. In addition to a fairly significant increase in the rate of tin chlorination with the increase in chlorine partial pressure, the data show two distinguishing features. The first is the extent of increase in tin chlorination with increase in the linear gas flow rate. At a gas flow rate of 4.0 mil/min one hundred percent conversion is obtained. The second notable feature is that the reaction initially proceeds at a high rate and then decreases rapidly after a few minutes at high conversions. For example 98 percent of conversion is obtained at the initial 10 minutes and one hundred percent of conversion after the next 10 minutes. The variation of the square of the weight of tin chlorinated per unit area with time for three different Cl2/N2 mixtures is shown in Figure 5 and Figure 6.
The experimental results follow the parabolic law. The observed parabolic rate constants KPare given in Table 2 and Figure 7 shows the dependence of the rate constant on the pressure in Cl2/N2 mixtures. Using the experimental data, it is mathematically possible to represent the results in the form of the following equations at 298 K and chlorine partial pressure range of 0.25 - 0.75 [MPa].
The rate constant increases linearly with the chlorine partial pressure. This result suggests that the chlorination of Tin is probably diffusion controlled at 298 K and the chlorination then proceeds at a steady state. Also, flow rates of 2 and 4 mil/min are very low and the dependence of KPon flow rate, as is shown in Figure 7, suggest that, at least with 2 mil/min the reaction is starved of chlorine. Also it may be that, with flow of 2 mil/min, back diffusion of SnCl4(g) occurs and prevents the attainment of 100 percent chlorination.
![]()
Figure 3. Effect of the partial pressure of chlorine on the tin extraction during chlorination in N2/Cl2 mixture at 298 K.
![]()
Figure 4. Effect of the partial pressure of chlorine on the tin extraction during chlorination in N2/Cl2 mixture at 298 K.
![]()
Figure 5. Variation of the parabolic rate constant with chlorine partial pressure at 298 K.
![]()
Figure 6. Variation of the parabolic rate constant with chlorine partial pressure at 298 K.
![]()
Figure 7. Effect of chlorine partial pressure on the tin chlorination at 298 K.
![]()
Table 2. Parabolic rate constant determined for the chlorination of iron scrap at 298 K under Cl2-N2 mixtures. Total pressure 0.1 [MPa].
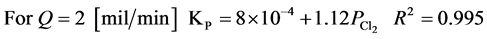 (1)
(1)
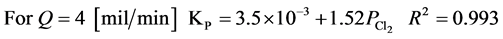 (2)
(2)
Because of the volatility of the reaction product, no scale is formed and the reaction product sublimes on the walls of the condenser as shown in Figure 8 and along the inner walls of the reaction tube. X-ray analysis of the surfaces of samples and the sublimate white powder were made. The surface of the samples showed no reaction products. Only tin or iron at lower yield and pure iron at one hundred percent yield were found.
Nine samples of the white powder were analyzed by X-ray and UV spectrophotometry. For X-ray analysis, the precipitate was sealed in a glass capillary tube to protect from atmospheric moisture, which was identified as SnCl2 of high purity, as shown in Figure 9.
Chemical analysis of the metal basis by UV spectrophotometry of the precipitate is showed in Table 3, the result of chemical analysis validate the X-ray diffraction analysis.
As illustrated in Table 3, the SnCl2(s) obtained in this work has the same high purity that of those produced by some commercial laboratories [28] - [34] .
![]()
Figure 8. Crystals of Sn(II) Cl2 deposited on the inner walls of the condenser.
![]()
Figure 9. X-ray diffraction pattern of samples of sublimated white powder.
![]()
Table 3. Chemical analysis of metals basis for some commercial Ti(II) chloride.
Visual observations made through a cathetometer showed the formation of a transparent liquid film during the reaction, and this liquid film was removed at the end of each run by volatilization into the pure dry nitrogen. Qualitative and quantitative chemical analysis showed that the liquid film was pure SnCl4(l). No indication of chlorine or stannic chloride reaction with pure iron was found.
The flow rates used correspond to small Reynolds number. Therefore, a laminar flow in the reaction tube was established and a laminar chlorine boundary layer at the surface of the sample is assumed. According to the experimental results, it may be assumed that the chlorination process consists of the following steps:
1) Transfer of chlorine molecules to the sample surface across the laminar boundary.
In this process the rate is flow dependent and increases with the Cl2 content.
2) Formation of liquid products due to a fast chemical reaction on the solid surface.
3) Formation of the volatile products.
4) Transfer of volatile products away from the liquid surface across the laminar boundary layer back to gas stream.
From the proposed mechanism, it would appear that in the initial stages, chlorination occur according to the following reactions.
Liquid film formation
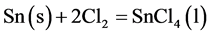 (3)
(3)
and the volatilization process
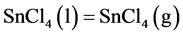 (4)
(4)
at the liquid film/gas interface. Then the weight loss of tin is caused mainly by the volatilization of SnCl4(l) and its diffusion through the chlorine boundary layer.
In the sublimation process from the gas phase, the following equilibria have to be considered
![]() (5)
(5)
The formation of pure SnCl2(s) is favored by decreasing the chlorine partial pressure, this happens because the flow of the gas mixture leaving the sample has been depleted free chlorine.
4. Conclusion
It has been demonstrated on a laboratory scale that it is feasible to separate tin from tin coated scrap as high purity SnCl2. The experimental results indicate that at the temperature of 298 K, ![]() of 0.75 [MPa] gas flow rate of 4 [mil/min] and chlorination time of 12 min can be achieved at a recovery of 100%. The reaction of tin coated scrap in nitrogen/chlorine gas mixtures, as illustrated by the proposed mechanism, provides unexpected possibilities for the manufacturing industry of advanced inorganic materials, because it provides a very inexpensive method for the manufacture of high purity stannous chloride. This product has many uses in the electronics field [35] , for example, as a precursor, from the preparation of high-quality magnetic and semiconducting materials and thermoelectric materials.
of 0.75 [MPa] gas flow rate of 4 [mil/min] and chlorination time of 12 min can be achieved at a recovery of 100%. The reaction of tin coated scrap in nitrogen/chlorine gas mixtures, as illustrated by the proposed mechanism, provides unexpected possibilities for the manufacturing industry of advanced inorganic materials, because it provides a very inexpensive method for the manufacture of high purity stannous chloride. This product has many uses in the electronics field [35] , for example, as a precursor, from the preparation of high-quality magnetic and semiconducting materials and thermoelectric materials.
Acknowledgements
The authors are sincerely grateful to Miss. Wang for her help to obtaining financial support for this work.
NOTES
![]()
*Corresponding author.