SIRT and Its Unresolved Problems—Is Imaging the Solution? A Review ()
Received 8 June 2016; accepted 9 July 2016; published 12 July 2016

1. Introduction
Hepatocellular Carcinoma (HCC) is, with over one million new cases worldwide per year, the most common primary liver cancer. The tumor stage and the level of liver impairment determine the possible treatment options. While a surgical resection of the entire tumor is ideal and provides the best chance for curing the patient, only about 20% percent of the patients are candidates for such a surgery. This is due to the fact that the disease is only diagnosed at an advanced stage [1] . In addition to primary liver cancer, other cancer types can metastasize into the liver. Colon and rectal cancer, which is the fourth most common cancer in the United States and Europe, is responsible for the majority of hepatic metastases [2] [3] .
Given the high rates of unresectable liver malignancies, the interest in and the importance of alternative treatment techniques progress. Standard techniques to treat unresectable liver malignancies during the last years include ablation and embolization techniques containing transarterial chemoembolization (TACE) for tumors at an intermediate stage and radiofrequency ablation (RFA) for tumors at an early stage [4] [5] . With the introduction of microspheres which have Yttrium 90 as an integral constituent, the interest in Selective Internal Radiation Therapy (SIRT) to treat hepatic tumors started. This paper deals with the development of SIRT in the past years as a novel and promising technique and has its main focus on the imaging modalities used in the treatment process.
Goal: The goal of this work is to give an overview of SIRT using Y90 microspheres. The main focus will be set on the upcoming limitations of the systems that are in use and possible solutions.
2. Selective Internal Radiation Therapy
Selective Internal Radiation Therapy is a minimal invasive catheter-based treatment option for liver tumors. Using Yttrium 90 as the radioactive substance ionizing radiation is directly delivered to the tumorous tissue by microspheres placed into the hepatic artery. The Y90 radioembolization treatment combines the advantages of internal radiation therapy and the embolic effect of Y90 microspheres. The importance of the Y90 therapy arises from the limited treatment options available for unresectable hepatic malignancies [4] .
2.1. Background
The use of external beam radiation to the entire liver to treat hepatic malignancies is restricted. The radiation dose tolerated for patients with non-affected liver function is limited to 30 Gy. This is below the dose required to damage the tumor sufficiently, which is suggested to be around 95 Gy [4] [6] .
The minimal invasive catheter-based placement of Y90 microspheres into the hepatic artery allows for specific internal radiation in direct proximity of the tumor. Yttrium-90 is a β-emitter with a small γ-component and has a mean tissue penetration of 2.5 mm. Therefore, Y90 microspheres can deliver radiation doses of 50 to 150 Gy directly to the tumor, given that the majority of the radiation spares healthy liver tissue and only affects the malignant tumor region [5] .
The basic principle behind the intra-arterial placement of microspheres arises from anatomic and physiological aspects of the liver tissue. The blood supply of the liver tissue is delivered by the portal vein and the hepatic artery. The technology takes advantage of the fact that healthy liver tissue derives over 70% of its blood supply from the portal vein, whereas the tumorous tissue obtains the majority of its blood supply from the hepatic artery (90%). Placing the microspheres inside the hepatic artery minimizes harmful effects on the normal liver parenchyma, but delivers radiation directly to the tumor [4] [5] [7] .
2.2. Treatment Procedure
Selective Internal Radiation Therapy does not only consist of the injection of microspheres itself, but includes necessary multidisciplinary imaging modalities [8] . The interaction of preoperative and postoperative parts included in the treatment procedures give an overview of the complex therapy option and will gain its importance when the limitations of the technique are discussed later in this paper.
Preoperative
After a patient is diagnosed with unresectable liver cancer the appropriate further treatment is discussed. Treatment results will strongly depend on the choice of the right therapy option. To ensure good results when performing Y90 radioembolization treatment an appropriate patient selection is required [9] . Following preoperative procedures have to be performed to estimate the risks of the treatment and to evaluate any possible contraindications:
A) Performance Status
A physical examination is conducted to determine the performance status of the patient. This includes serum chemical analysis to evaluate the liver function and to determine known tumor markers. Studies have shown that patients with a poor performance status have a high risk of liver failure during the treatment [4] . Physicians decide on an individual basis, if the performance status is a contraindication for Y90 radioembolization or not. Each patient requires special consideration weighting further favorable factors. Patients with a poor performance status may also be suitable for Y90 therapy. In general it can be said that patients with irreversible elevations in serum bilirubin, which points to a severely damaged liver function or a disseminated extra-hepatic malignant disease, are not suitable for the therapy with Y90 microspheres. Patients chosen for Radioembolization should have a minimal live expectancy of three months [4] [10] [11] .
B) Angiogram
An angiogram is required to determine the status of the blood supply to the liver and to verify the appropriate placement of the catheter during the treatment. Performing Digital Subtraction Angiography (DSA) extrahepatic shunt vessel can be detected prior to the Technetium-99 macroaggregated albumin scan (Tc-99 MAA), explained in 2.2.E [1] [4] [12] [13] .
C) Liver Scan
A MRI or a CT scan of the liver is necessary to estimate the mass of the liver. This is essential to calculate the dosage required for the desired effect on the malignant tissue [14] [15] . Here, the CT scan provides the fastest and most reproducible method to determine the liver and tumor volume. Often a contrast injection CT scan follows the non-contrast CT scan for a better estimation of the tumor. An additional MR scan supports to determine the tumor volume and spread, because it provides a better soft tissue contrast [16] .
D) Dosimetry Planning
Applying internal radiation therapy always requires complex dosimetry planning to ensure that the desired target dose is reached. The planning differs between the various types of microspheres and has a major impact on a successful outcome of the procedure.
E) Technetium-99 macroaggregated albumin scan (Tc-99 MAA)
A Tc-99 MAA scan is performed to determine the amount of extrahepatic shunting that could occur during Y90 radioembolization treatment (see Figure 1). Shunting sometimes occurs in patients when part of the hepatic arterial blood supply bypasses the capillary bed and flows directly into the venous system. In these patients, a fraction of the injected microspheres would not be embolized in the hepatic artery but would be deposited into
![]() (a) (b)
(a) (b)![]() (c) (d)
(c) (d)
Figure 1. Result of a planar Tc-99 MAA scan with the regions of interest: liver (yellow) and lung (red) (a) with reference to the contrast-enhanced CT image in (b). Tc-99 MAA SPECT/CT for dosimetry planning with regions of interest: tumor (red), in-target normal liver (yellow) and out target normal liver (blue) (c) and a postoperative PET/CT (d) [20] .
the lungs. To analyze the risk Tc-99 MAA is injected through a hepatic artery catheter and a scintigraphy scan of the thorax and the abdomen is taken. This is used to determine the fraction of injected radioactive material that is delivered to the target tissue and the fraction which is shunted into the lungs (see Equation (1)) or is delivered via blood flow to the upper gastrointestinal tract. Y90 radioembolization is contraindicated if the fraction of lung shunting is over 20% and if the backflow to the gastrointestinal tract cannot be corrected using catheterization methods (balloon catheterization or coiling) [4] [6] [17] [18] .
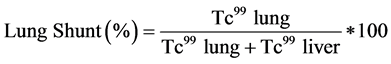 (1)
(1)
Extrahepatic shunting is one of the main risks in SIRT; its occurrence causes several side effects and severe problems following the procedure. Although the Tc-99 MAA scan provides a good approach to estimate the shunting, its application is restricted. New personalized methods to detect dosimetry distribution improve the procedure outcome [19] , but Tc-99 MAA particles are irregular in size and differ from the shape of the microspheres used for radioembolization. Therefore the Tc-99 MAA provides no direct conclusion on the microsphere distribution after the injection [13] [16] .
Intraoperative
After weighting all risks, excluding the contraindications and performing the additional preoperative tests, the actual injection of the microspheres can be performed. The Y90 microspheres are selectively injected by percutaneous access to the femoral artery and are guided to the hepatic artery by fluoroscopy X-Ray imaging. The intra-arterial catheter based placement of the microspheres can be done in a local (segmental), regional (right or left hepatic artery) or whole-liver (proper hepatic artery) treatment. The catheter must be placed such that it does not occlude the vessels in which it is placed. The exact position for the placement of the microspheres has to be evaluated using the preoperative techniques and depends on the tumor size and position. Using Y90 microspheres it is not possible to depict the exact placement of the microspheres intraoperatively. During the precise placement of the microspheres the patients are sedated and the injection time runs between 2 and 4 hours [1] [6] [21] .
Postoperative
Directly after the delivery of the microspheres a Single Photon Emission Computed Tomography (SPECT or SPECT/CT, when combined with a CT scanner) scan is acquired to evaluate the placement and position of the microspheres. It is useful to compare this scan with the results of the Technetium-99 macroaggregated albumin scan to determine the difference between the scans and the actual distribution of the microspheres. This gives special advantages for the planning of a possible retreatment. Post-treatment evaluation using SPECT is limited due to bad resolution of the image caused by a low count rate of Bremsstrahlung [16] [22] . Therefore recent studies and the development of new Positron Emission Tomography (PET)/CT scanners with time-of-flight technology introduced the possibility of a PET/CT scan to evaluate the microsphere distribution. Although Yttrium 90 is a β-emitter it produces 32 positrons per million decays and with that enables a PET scan. This provides better contrast and resolution and might also distinguish necrotic parts within the tumor area [22] - [24] .
If no unexpected side-effects occur then patients are discharged from the hospital on the day of treatment, as there is no medical constraint for radiation safety in combination with the Y90 microspheres. A follow-up exam is necessary to evaluate the success and the effects of the injection. This is usually done after 30 days using imaging methods and laboratory tests to determine the degree of tumor shrinkage. A post-operative diffusion weighted imaging (DWI) MRI can be used as an early imaging marker to evaluate the output response [25] . Further postoperative observation occurs at three-month intervals. A second and third treatment with Y90 microspheres is recommended after 4 - 6 weeks, if a positive result was achieved during the first session [1] [26] .
The overall SIRT workflow is described in Figure 2 and an example for the multimodal imaging approach is shown in Figure 3 [27] .
3. Y90 Microspheres
Currently all systems commercially used for the Selective Internal Radiation Therapy have Yttrium 90 as the radioactive substance to deliver ionizing radiation to the tumorous tissue.
Yttrium 90: The physical properties of Yttrium-90 make it well suited for the medical treatment of liver malignancies. It is a pure β-emitter and decays to Zirconium-90 with a half-life of 64.1 hours. It has a mean tissue
![]()
Figure 2. General overview of the SIRT procedure.
penetration of 2.5 mm and a maximum penetration of 1 cm. Yttrium-90 is the product of the β-decay of Strontium-90. The maximum energy released is 2.27 MeV with a mean of 0.93 MeV. Although Yttrium-90 is a β-emitter, secondary radiation in the form of γ-radiation as an effect of Bremsstrahlung and positrons can occur [5] [28] .
Since the 1960s Yttrium-90 has been used to treat hepatocellular carcinoma in patients. Initially, metallic particles of Yttrium-90 oxide (Y-902O3) were injected intraarterial into the human body. In 1987 the first animal studies with glass microspheres were tested. Yttrium-90 incorporated in glass matrices solved the problem of leaching, which occurred during the implementation with metallic particles. Nowadays Y90 can be delivered to hepatic malignancies as either an integral constituent of glass microspheres or as a resin-based microsphere. Two commercially available systems are approved and explained and described in the following chapter [21] .
3.1. TheraSphere®
TheraSphere® microspheres (MDS Nordion, Canada) are insoluble glass microspheres which have Yttrium-90 as an integral constituent of the glass. Figure 4 shows a microscopic view of Thera Sphere® microspheres.
![]()
Figure 4. Microscopic view of Yttrium-90 microspheres beside a strand of hair [1] .
Each sphere has a diameter between 20 and 30 μm and contains approximately 2,500 Becquerel (Bq). TheraSphere ® microspheres are supplied in a vial of 0.6 ml sterile, pyrogen free water and are shielded with a 12 mm thick acrylic shield. One milligram of TheraSphere® contains 22,000 to 73,000 microspheres. Thera- Sphere® microspheres are available in six different activity sizes at calibration (3, 5, 7, 10, 15 and 20 GBq). To obtain the different activity sizes the number of microspheres in one vial varies from 1.2 to 8 million. This is not sufficient to cause a significant blockage in the main hepatic artery, therefore, the effect of complete embolization does not occur. The cost of TheraSphere® in the US market is 13,000 US$. This price does not include treatment administration and follow-up treatment. [1] [4] [29] .
Dosimetry
Dosimetry planning for Y90 radioembolization using TheraSphere® follows the so called partition model. The injected doses are based on the desired dose to the target mass. The recommended dose to treat liver malignancies is between 80 and 150 (mostly 120) Gy. Equation (2) describes the calculation of the activity A needed to acquire the desired dose.
 (2)
(2)
where D is the desired dose in Gy and M the estimated mass of the liver which is determined using imaging methods such as MRI or CT scans. It is common to estimate the liver volume using a CT scan and then convert it to the mass using the conversion factor 1.03 kg/ml. To determine the exact radiation dose delivered to the liver after the injection, the lung shunt fraction (F) has to be taken into consideration [4] [14] [15] :
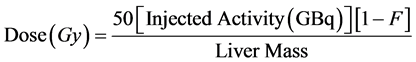 (3)
(3)
TheraSphere® is indicated for the radiation treatment in patients suffering from unresectable liver malignancies [17] .
3.2. SIR-Spheres®
SIR-Spheres® (Sirtex Medical Limited, Lane Cove, Australia) represent another type of microspheres used for Y90 radioembolization. They consist of biodegradable resin-based microspheres containing Yttrium-90 with an average size of 35 μm (range 20 - 60 μm). SIRSpheres® only come with the dose size of 3 GBq and are provided in a vial of 5 ml sterile water. Each vial contains 40 to 80 million microspheres and is calibrated to deliver 3 GBq of Y90 on the day of usage. The corresponding activity per microsphere is approximately 50 Bq. Given the lower dose rate available when using SIR-Spheres® in comparison to TheraSphere® a higher amount of microspheres must be delivered to the patient to obtain the desired target dose. This, in addition to the larger diameter of the resin-based microspheres often leads to a saturation of the vascular bed with microspheres. Using SIRSpheres® an embolic status can be reached, which can block the blood supply in the hepatic artery. Therefore the fluoroscopic guidance is especially important for the treatment using SIR-Spheres® because the injection has to be stopped as soon as the embolic status is reached [1] [4] [28] .
Dosimetry
The dosimetry calculation for SIR-Spheres R varies from TheraSphere® radiation treatment. Two different methods based on the activity and not on the target radiation dose can be used to perform the dosimetry planning for SIR-Spheres® [4] [17] [28] [30] :
A. Empirical model
This method is based on a broad estimate of tumor infiltration and the recommended dose for the treatment is obtained from Table 1 [4] :
B. Body Surface Area Model
This method uses the body surface area (BSA) as an estimation for the liver volume in combination with the tumor infiltration of the liver. It provides a more objective calculation of the required activity and is therefore the preferred model to use. The required dose calculation follows Equation (4) [30] :
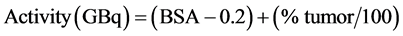 (4)
(4)
For both models the recommended dose has to be modified if significant lung shunting occurs or a partial liver treatment is planned for a local or regional tumor treatment. Therefore, specific recommended multiplication factors are available in package-inserts of SIRSphere® [30] . Table 2 provides a final summarizing comparison of the differences between the two microsphere systems used.
Within the development of the microspheres TheraSphere® was first approved for the treatment of HCC and SIR-Spheres ® for the treatment of colorectal liver metastases [17] . In current practice both types of microspheres are used as a treatment option of both types of liver malignancies. Newer studies introduced Y90 Radioembolization as a promising procedure in the treatment of liver metastases from neuroendocrine tumors [31] .
3.3. Clinical Results
Different clinical studies concerning the efficacy and safety of Y90 radioembolization treatment have been done in the past. Analysis and evaluation of these studies have been proven to be difficult due to different types of microspheres used, low number of patients and heterogeneous evaluation criteria. Nevertheless the following section attempts to sum up clinical results of Y90 radioembolization for hepatic malignancies. The efficacy and safety of radioembolization in general and in comparison to other treatment options is evaluated.
![]()
Table 1. Dosimetry calculations using the empirical model.
![]()
Table 2. Comparison of Y90 radioembolization systems.
Y90 Radioembolization for unresectable hepatic malignancies
Saxena et al. (2014) [32] compared and evaluated the results of 20 different studies including 979 patients who were treated for unresectable colorectal liver metastases with either glass or resin-based microspheres. Their results are summarized in Table 3. Most of the patients who were chosen for Y90 radioembolization had undergone several lines of chemotherapy before the treatment with microspheres was started. There is also a not negligible number of patients who were previously treated with hepatic resection, ablation or other therapies including transarterial treatments. Transarterial Chemoembolization (TACE) which was used in the past as the standard treatment for unresectable hepatic malignancies which were insensitive to chemotherapy. Y90 radioembolization was generally chosen when resection or ablation was not possible and transarterial chemoembolization did not lead to the desired effect. This group of patients naturally has a relatively poor prognosis.
A complete response of the tumor to Y90 radioembolization was very rarely seen. About a third of the patients have shown a partial response and on average 40.5% had a stable disease after treatment. Only 17.5% had a progressive disease after the injection of microspheres. The response rates concerning Y90 radioembolization are comparable with those from TACE treatment. There has been no significant difference mentioned between results of treatment with microspheres based on glass or resin.
Table 4 shows that about a half of the patients developed symptoms related to acute toxicity caused by the microspheres. Values here varied between 11% up to 100% in the different studies. The discrepancy between the different studies is high. Some mentioned fatigue and abdominal pain as complications. Others did not see those symptoms as complications. Symptoms that emerged were fatigue, abdominal pain, vomiting and gastritis. The majority was transient and could be solved without any active intervention. These complications are related to the therapy and are summarized as the postradioembolization syndrome. The incidence of patients developing delayed toxicity complications was low (5%). The most common delayed complications have been liver dysfunction including liver failure, gall bladder complications and gastritis. Bennink et al. pointed out that more SIRT session can minimize the risk for a decreased liver function after the complete procedure, where the time between two sessions counts as the recovery time [33] .
![]()
Table 3. Summary of response of patients with CRCLM undergoing Y90 therapy.
![]()
Table 4. Side effects caused by Y90 radioembolization.
In general, the rate of complications after Y90 therapy did not increase in comparison to complications occurred after radiofrequency ablations or transarterial chemoembolization. The authors of the study concluded that Y90 therapy is safe and effective for the treatment of colorectal hepatic metastases [32] .
Although the study by Saxena et al. (2014) only covered colorectal liver metastases, the results can be generally transferred to the treatment of hepatocellular carcinoma. Salem et al. (2010) [34] observed the treatment of HCC with TheraSphere® in 291 patients. Portal vein thrombosis was not considered as an exclusion factor. Response rates and side effects that occurred were comparable to the study of Saxena et al. (2014), but the main goal of the study was to determine the interaction between specific tumor factors and the survival outcome. They concluded that an advanced disease leads to lower response rates and a reduced time-to-response. Multifocal tumor distribution was found to be associated with a poor prognosis, whereas, portal vein thrombosis and extrahepatic metastases had no significant influence. The authors concluded that the relatively small group of patients often limits the significance of the studies. Further studies are necessary to define specific prognostic factors and to make a comparison of the influences of those factors between other loco regional therapies (radiofrequency ablation and TACE) [34] .
Y90 radioembolization vs. TACE
Both Y90 radioembolization and Transarterial Chemoembolization (TACE) are transarterial treatment options for liver malignancies. During the last years TACE has become the standard treatment for unresectable hepatic malignancies (at an intermediate tumor stage), but with the development of Y90 microspheres the significance of radioembolization started to increase.
Salem et al. (2011) [35] performed a comparative analysis of patients treated with Y90 radioembolization (123 patients) and TACE (122 patients). Results of the study showed that Y90 radioembolization and TACE lead to similar survival of the patients after the treatment. However, Y90 radioembolization induced a prolonged time-to-response in comparison to the traditional method, which was performing TACE treatment. This cannot directly be related to the survival time but is of high interest, if the treatment is used as a bridge to resection or transplantation [36] . Additionally three main advantages of Y90 radioembolization were observed. First, it is especially suitable for elderly patients because a postembolization syndrome, which is often mentioned in combination with TACE, does not occur. This leads to reduced abdominal pain. The postembolization syndrome usually requires a hospitalization of patients after the treatment with TACE. Whereas, Y90 radioembolization patients are usually released on the day of treatment. Finally, portal vein thrombosis is a contraindication for TACE but given the lower embolic effect of microspheres, Y90 radioembolization offers a new option for patients with compromised portal flow. Patients with or without portal vein thrombosis can be treated. Portal vein thrombosis is listed as a contraindication in package inserts for both types of microspheres. [14] [28] . Studies showed that microspheres can be injected in patients with portal vein thrombosis weighting the risks using the Tc-MAA scan [34] . The authors of the study concluded that Y90 radioembolization can be used safely and provides a possible alternative to TACE with similar survival outcome [35] [37] . Y90 radioembolization as well as TACE are considered to be palliative treatment options, whereas liver transplantation and tumor resection count as the only curative treatment procedures. Nevertheless radioembolization as well as TACE can provide a bridge to the curative methods with downstaging the liver tumor [13] .
Combining Treatments
Treatment effectiveness (tumor response and time-to-disease progression) can be increased when combining systemic chemotherapy with Y90 radioembolization
The combination of Y90 microspheres in combination with Sorafenib®, which is an oral multikinase inhibitor, is widely spread. Sorafenib® reduces tumor cell proliferation and is a drug used to expand the life span of a patient or as a bridge to liver transplantation, and it is often used in an advanced stage of the disease. Since Sorafenib® in combination with TACE leads to conflicting results it was hoped that Sorafenib® has no negative effect on Y90 radioembolization.
A first randomized study concluded that on one hand lower radiation doses for Y90 microspheres were required after the additional use of Sorafenib®. But on the other hand a possible transplantation of the liver after pretreatment failed more often when using Sorafenib® and Y90 microspheres together [11] [23] [38] .
3.4. Limitations
Y90 radioembolization is currently limited to palliative treatment. A complete cure or healing of the patients is extremely rare. Therefore Y90 radioembolization is used to improve quality of life and extend the time of survival. In addition it is used as a bridge to resection, transplantation or ablation procedures.
Portal vein thrombosis was seen as a contraindication for Y90 radioembolization in the past. But several studies have now concluded that the use of Y90 microspheres is not limited in patients with portal vein thrombosis. Accurate imaging of the microspheres would allow better evaluation of the possible impact of portal vein thrombosis and could better predict the possible treatment outcome. A main problem for a successful procedure outcome is extrahepatic shunting, the use of Tc-99 MAA scans in addition to DSA improved the results. The difference between Tc-99 and Yttrium 90 particles still causes problems in the evaluation of the fraction of extrahepatic shunting. Real-time imaging of the injection procedure is impossible, therefore the exact positions of the microspheres cannot be determined and a direct reaction by surgeons on the placement is not possible. Once the microspheres are injected it is not possible to remove them. The guidance is difficult and imaging is necessary to confirm the right placement. Pre- and postoperative methods are essential to optimize the injection of the microspheres. Postoperative SPECT scans can only confirm the position of the microspheres qualitative but a quantitative evaluation is impossible due to the low amount of emitted Bremsstrahlung. The introduction of PET/CT scans improved the postoperative evaluation methods but the depiction of the complete distribution of microspheres is still limited. It is of high importance to develop possibilities of an intraoperative detection of the microspheres and an improved postoperative evaluation [16] .
4. Holmium 166 Microspheres
A new approach to overcome the limitations of SIRT using Yttrium 90 is the development of 166 Holmium-poly(L-lactid acid) microspheres (Quirem Medical BV, The Netherlands, Utrecht), with a half-life of 26.8 h.
These systems are commercially not available, but first clinical trials introduced promising results. The mean diameter of Holmium based microspheres is 30 μm (20 - 50 μm) and around 600 mg of nonradioactive Holmium is used for each treatment. Before the treatment procedure the microspheres have to be activated by neutron radiation. Although Holmium 166 is, like Yttrium 90, a β-emitter, its application has several advantages. The amount of secondary emitted γ-radiation, Bremsstrahlung, is significantly higher (E = 81 keV) than with Yttrium 90. This makes Holmium 166 well suited for SPECT scans and improves the depiction of microsphere distribution and delivered dose estimation. The use of a scout dose can replace Tc-99 MAA for the prediction of extrahepatic lung shunting; this was restricted because of the differences in shape and size. In addition to its higher amount of Bremsstrahlung, Holmium 166 is paramagnetic and can therefore be seen in MRI. The use of Brems- strahlung for a SPECT scan and MR-imaging could provide a quantitative description of microsphere distribution [31] [39] - [41] .
Dosimetry
Smits et al. [40] performed a phase 1 clinical trial dose-escalation study and estimated that 60 Gy should be the aimed whole-liver absorbed dose for clinical 166 Holmium radioembolization. The acquired amount of 166 Holmium radioactivity can be calculated as following:
![]() (5)
(5)
With the liver weight (LW) determined using contrast-enhanced CT images and the aimed whole-absorbed liver dose (LD). The introduction of the use of a scout dose (60 mg/250 MBq) after Tc-99 MAA treatment increases procedure safety and is estimated to have the ability to completely replace Tc-99 MAA pretreatment scans [39] - [41] .
Smits et al. [41] were able to describe the dosimetry distribution after injection quantitative using SPECT- and MR-imaging. Van de Maat et al. showed the biodistribution of 166 Holmium microspheres with MRI-based concentration maps (Figure 5) [42] . These results allow to hope for a better controllable and more accurate treatment using SIRT. A summary of the properties of Holmium 166 microspheres is given in Table 5.
Limitations
166 Holmium-poly(L-lactid acid) microspheres are not yet approved by the Food and Drug Administration (FDA) and European Medicine Agency as a medical device and are therefore not commercially available. Its
![]() (a) (b) (c) (d)
(a) (b) (c) (d)
Figure 5. (a) MRI-based 166Holmium concentration map (b) MRI based absorbed dose map together with SPECT images (c) and MR T1 weighted images (d) [42] .
![]()
Table 5. Summary of Holmium 166 microspheres.
use is restricted to clinical trials and for research applications. Cosimelli et al. [31] pointed out, that more severe side effects using Holmium 166 based microspheres in comparison to Yttrium 90 microspheres have been described.
A clinical application of 166 Holmium microspheres could be more complex in practice, due to is lower half- life (26.8 h compared with 64.2 h of Yttrium 90) [31] [39] - [41] .
5. Conclusions
SIRT is the transarterial injection of microspheres into the hepatic artery to treat unresectable liver malignancies.
It is mainly used for the treatment of unresectable hepatocellular carcinoma or unresectable colorectal liver metastases. Multidisciplinary imaging plays an important role in SIRT, where the accurate depiction of microspheres is the main challenge. Pre- and post-operative image evaluation helps to reach a better procedure outcome. A treatment with Yttrium 90 microspheres is considered to be safe and efficient. Currently two different systems are in use, TheraSphere® and SIR-Spheres®, which are based on either glass or resin microspheres. Clinical results reported no significant difference between both systems and the liver malignancy they are intended to treat. Comparison studies between Y90 radioembolization and TACE showed no significant differences in survival outcome of the patients, but a decreased amount of side effects in combination with the injection of the microspheres. Y90 microspheres cannot be detected during their injection and also post-treatment evaluation is difficult, which is the most important limitation arising from Y90 radioembolization treatment. The recent increased use of PET scans in postoperative evaluation has already improved the treatment procedure, but with the introduction of 166 Holmium-poly (L-lactid acid) microspheres completely new imaging possibilities in SIRT arise. The use of a scout dose to replace Tc-99 MAA scans and the use of SPECT and MR imaging to calculate quantitative dose distribution to enable personalized patient treatment are the main advantages of these new microspheres. Shortfalls which might arise are more severe side effects and their practical appliance. More clinical trials with 166 Holium have to be performed to assess safety and efficiency. General improvement of SIRT might be its introduction as a first-line treatment to treat tumors with radioembolization as soon as possible.
In general, a multimodal imaging approach for the whole SIRT procedure is important to gain optimal treatment results.
6. Future Outlook
The main challenge for future research on SIRT is to overcome the limitation of image guidance. The use of new substances like 166 Holmium is a first step, but its practical appliance still has to be tested and approved. Real-time image guidance of microspheres could depict them intraoperatively, which might optimize the entire treatment procedure. Due to its paramagnetic properties 166 Holmium could be a candidate for intraoperative monitoring, e.g. during interventional MRI. To introduce interventional MRI injection of microspheres into clinical practice, a lot of additional research and testing has to be done. Here, the general improvement and introduction of MRI interventions are an important factor. Recently, a hand-held γ-camera system was introduced [43] . With this system it is possible to combine radioactivity distribution and tracking information. This approach might be a novel method to intraoperatively depict microspheres. Given the fact that Yttrium 90 based microspheres and Holmium 166 based microspheres come with different advantages, a combination of both could be considered. It has to be tested in future if may lead to a better procedure outcome or make delivery of microspheres and dose calculations much more complicated.
Furthermore, clinical studies should be extended to enable statistically significant evaluation of SIRT. Here, it might be of interest to better compare the different microspheres which are in use. In addition, a comprehensive comparison between Y90 radioembolization and TACE could be of interest, and also a combination of SIRT with chemotherapy [44] . An improvement of the clinical studies could enable a better selection of the patient to optimize the treatment procedure. Additionally, prognostic factors which determine the possible outcome should be determined. This could further individualize and improve the therapy by selecting the most promising treatment option for the patient.