Study of the Dielectric Behaviour of Cr-Doped Zinc Nano Ferrites Synthesized by Sol-Gel Method ()
Received 11 May 2016; accepted 14 June 2016; published 17 June 2016

1. Introduction
Ferrites have paramount advantages over other types of magnetic materials due to high electrical resistivity and low eddy current losses [1] [2] . For the most favorable combination of low cost, high stability, high quality and lowest volume, ferrites are considered to be the best core material choice over a wide range of frequency [3] . Among all magnetic materials, ferrites are the most useful because in addition to magnetic properties, they are also good electrical insulators. The dielectric properties of ferrites are dependent on several factors, such as the method of preparation, heat treatment, sintering conditions, chemical composition, cation distribution, pH, nature and type of substituent, the ratio of Fe3+/Fe2+ ions, frequency and crystallite size [4] - [6] . The important parameters for any dielectric substance are its dielectric constant or dielectric permittivity (ε'), which is the ability to store charge in a capacitor and dissipation factor (D) which is a measure of the energy dissipation in the material. Extrinsic losses are associated with the crystal defects, e.g. porosity, grain boundaries, micro-cracks, random crystal orientations, and impurities, etc. It is evident from the previous studies [7] that the losses in sintered polycrystalline ceramics are greatly affected by these extrinsic factors. All these parameters can play a key role in the modification of the dielectric behavior of spinel ferrites which can be more useful for the desired applications.
ZnFe2O4 has the normal spinel structure [8] in which Zn2+ ions occupy the tetrahedral sites, and all the Fe3+ ions occupy octahedral sites. ZnFe2O4 exhibit good dielectric and magnetic properties; hence these ferrites have a great importance from the application point of view, where these are widely used in many ferrite devices and production of electronic and magnetic components, converters, and electromagnetic wave absorbers [9] .
Polycrystalline ferrites are very good dielectric materials which have numerous applications at microwave frequencies. The study of dielectric properties gives valuable information about the behavior of localized electric charge carriers and can explain the phenomenon of dielectric polarization and electrical conduction in the material. The experimental conditions used in the preparation of these materials show a strong impact on the properties of the resultant ferrite nanoparticles. For this reason, several methods have been used in the preparation of nanoparticles, like the co-precipitation method [10] [11] , sol-gel technique [12] - [14] , hydrothermal method [15] [16] , microwave sintering method [17] , spray-spin-heating-coating method [18] and auto combustion method [19] . Out of all these, sol-gel method is promising technique for the synthesis of nano ferrites in bulk scale due to the production of homogeneous particles. The sol-gel method is the most convenient technique to synthesize nanoparticles because of its simplicity, inexpensive precursors, short preparation time, better control over crystallite size and other properties of the materials [20] . The current effort has been focused on studying the composition and frequency-dependent dielectric properties of Cr-Zn ferrites synthesized through sol-gel technique.
2. Experimental
2.1. Synthesis
Mixed Cr-Zn ferrites having the general formula CrxZnFe2−xO4 (where x = 0.0, 0.1, 0.2, 0.3, 0.4 and 0.5) were prepared using sol-gel method. The detailed structural analysis and magnetic properties of the synthesized Cr-Zn nano ferrites samples are reported in our earlier journal [21] . The synthesized powder was pre-sintered at 900˚C for 3 hrs and cooled slowly to room temperature. The pre-sintered samples were mixed with an organic binder (small quantity of polyvinyl alcohol (PVA)). The mixture was dried and pressed into disk-shaped pellets of 10 mm diameter and 2 mm thickness by applying a pressure of 3 tons. The samples were sintered again at 950˚C for 5 hrs and slowly allowed to cool naturally. For dielectric measurements, silver paint was applied on both sides of the pellets and air dried to have good electrical contact.
2.2. Dielectric Measurements
The dielectric measurements were carried out at room temperature using LCR Meter (Waynekerr Model: 43100) over the frequency range 100 Hz to 1 MHz. The values of capacitance (C) and dissipation factor (D) were noted directly at different frequencies.
The dielectric constant (ε') was calculated using the relation [22]
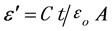 (1)
(1)
where C is the capacitance of the sample, t is the thickness; A is the surface area, and εo is the permittivity of free space.
From dielectric constant and dissipation factor (D), the ac conductivity (σac), of the ferrite samples can be calculated using the relation [23]
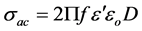 (2)
(2)
where ω = 2Πf is the angular frequency.
3. Results and Discussion
3.1. Dielectric Properties
The dielectric properties of Cr-Zn ferrites have been examined as a function of composition and frequency. Dielectric studies of these samples may be useful for widening its range of applications.
3.1.1. Frequency Dependence of Dielectric Constant (ε')
The frequency dependence of the dielectric constant (ε') for CrxZnFe2−xO4 spinel ferrite systems (where x = 0 - 0.5 in steps of 0.1) was studied at room temperature in the frequency range of 100 Hz to 1 MHz. Figure 1 displays the variation of dielectric constant (ε') as a function of frequency at room temperature. It can be observed that all the samples show strong frequency dependent. The dielectric constant decreases exponentially with increase in frequency, which is very rapid at lower frequencies and slower at higher frequencies. As frequency further, increase dielectric constant become almost independent of frequency. This is a normal dielectric behavior observed in most spinal ferrites [24] - [26] . It can be explained by the Maxwell-Wagner interfacial type
![]()
Figure 1. Variation of dielectric constant with frequency for CrxZnFe2−xO4 (x = 0.0 to 0.5).
polarization, which is also in agreement with Koop’s phenomenological theory (Koops, 1951) [27] [28] . According to this model the ferrite composed of good conducting grains separated by poorly conducting grain boundaries. On the application of electric field, the electrons reach the grain boundary through hopping, and if the resistance of the grain boundary is high enough, electrons pile up at the grain boundaries and produce polarization. However, as the frequency of the applied external field is increased beyond a certain value, the hopping frequency cannot follow up the field variation. It decreases the probability of the electrons reaching the grain boundary and as result polarization decreases which in turn causes to the decrement of dielectric constant.
The large value of dielectric permittivity (ε') at low frequency is due to the predominance of Fe2+ ions, oxygen vacancies, grain boundary defects, etc., while the decrease in ε' with frequency is due to the lagging of species contributing to polarizability behind the applied electric field. At the higher frequencies ε' remains constant which is attributed to the contribution of electric polarizability only.
3.1.2. Variation of Dissipation Factor (D) with Frequency
The variation of dissipation factor (D) against frequency at room temperature is depicted in Figure 2 for CrxZnFe2−xO4 where x = 0.0 to x = 0.5. All the samples exhibit an abnormal behavior of peaking. According to the Iwauchi [29] , there is a strong correlation between the conduction mechanism and dielectric behavior of the ferrites. The exchange of electrons between ferrous ions (Fe2+) and ferric ions (Fe3+) on the octahedral site may lead to local displacement of electrons in the direction of the applied field, and electrons determine the polarization. The dielectric loss in ferrites mainly originates due to the electron hopping and defect dipoles. The electron hopping contributes to the dielectric loss only in the low-frequency range. The response of the electron hopping is decreased with increasing frequency, and hence, the dielectric loss decreases in the high-frequency range. In the meanwhile, dielectric loss peak can be seen in Figure 2 for all the Cr-Zn ferrite samples. The appearance of the relaxation peak can be explained according to Debye relaxation theory [30] . The loss peak can be observed when the applied electric field is in phase with the hopping frequency in dielectric materials. The condition for observing a maximum in dielectric loss of dielectric material is given by
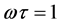 (3)
(3)
where ω = 2Πf, f is the frequency of the applied electric field, and τ is the relaxation time. The strength and frequency of the relaxation depend on the characteristic property of dipolar relaxation.
![]()
Figure 2. Variation of dielectric factor (D) with frequency for CrxZnFe2−xO4 (x = 0.0 to 0.5).
3.1.3. Variation of AC Conductivity with Frequency
Figure 3 shows the ac conductivity of Cr-Zn ferrite system sintered at 950˚C for 5 hrs. Study of ac conductivity of the samples has been performed in the frequency range of 100 Hz to 1 MHz. All the curves exhibit the significant dispersion with frequency, which is an important behavior of ferrites. The electrical conductivity in ferrites is mainly due to the hopping of electrons between the ions of the same element presented in more than one valence state and distributed randomly over crystallography equivalent lattice sites [31] . On the application of ac electrical field, this hopping of electrons gets boosted up, resulting in increasing in ac conductivity. The increase in the frequency of the applied electric field enhances the hopping phenomenon. Hence, there is an increase in ac conductivity.
3.1.4. Composition Dependence of Dielectric Behavior
The dielectric constant, dissipation factor and ac conductivity of Cr-Zn ferrite system have measured at a fixed frequency of 1 kHz. The values are recorded in Table 1. It is evident from the table that, all the dielectric parameters are greatly affected by Cr-content (x). The dielectric constant increases for all the Cr-substituted Zn-fer- rite samples except CrxZnFe2−xO4 (x = 0.4) sample. The variation of dielectric constant as a function composition is shown in Figure 4.
Cr-doped Zn-ferrites have higher conductivity than undoped ones. The increase in ac conductivity with the substitution of chromium may be due to the increase in Fe2+ ions at tetrahedral sites, which can increase the hopping of charge carriers between the Fe2+ and Fe3+ ions, and hence, there is an increase in ac conductivity of the Cr-Zn ferrites [32] .
![]()
Figure 3. Variation of ac conductivity with frequency for CrxZnFe2−xO4 (x = 0.0 to 0.5).
![]()
Table 1. Dielectric parameters of CrxZnFe2−xO4 (x = 0.0 to 0.5) at 1 kHz.
![]()
Figure 4. Variation of dielectric constant (ε') as function of Cr content (x) for Cr-Zn nanoferrite samples at 1 kHz frequency.
Figure 2 shows that the height and width of the relaxation peaks have increased unevenly in all the Cr-doped zinc ferrite samples. The increase in peak height with the substitution of Cr3+ ion is due to the increase in conductivity of the sample arising due to the increase of the Fe3+/Fe2+ ions available for the conduction process. The increase in the peak width is due to the strengthening of the dipole-dipole interactions which hinders the dipole rotation. From Figure 2, it is also important to note that the relaxation peaks of all the Cr-substituted Zn-ferrite samples except Cr0.2Zn∙Fe1.8O4 and Cr0.3Zn∙Fe1.7O4 samples are shifting towards lower frequency side with an increase in Cr-content. The strength and frequency of the relaxation depend on the characteristic property of dipolar relaxation. From the Table 1, it is clear that the Cr-Zn ferrites possess low dielectric losses. The low dielectric loss makes these samples especially attractive for high-frequency applications.
4. Conclusion
Cr doped Zn-ferrites were synthesized by sol-gel method. The dielectric properties have been examined as a function of frequency and composition. The room-temperature dielectric constant decreasing rapidly with increasing frequency indicates the normal dielectric behavior for all the samples. All the substituted samples have higher dielectric constant than the basic Zn-ferrite composition without chromium. Relaxation peaks were observed for all the samples in dissipation versus frequency curves and this relaxation peak is shifting to low frequency side with the substitution of Cr content (x). The ac conductivity was found to increase with increase in frequency and Cr concentration. The incorporation of Cr3+ for Fe3+ ions results in a significant impact on the dielectric behavior of the Cr-Zn ferrite system.
NOTES

*Corresponding author.