Received 30 December 2015; accepted 20 March 2016; published 24 March 2016

1. Introduction
Chile is considered as a sub-centre of the origin of cultivated potatoes, with native and introduced genetic material co-existing in the country. This accounts for its rich heritage, which should be characterised, conserved and used. Native potatoes present a rich variety of colours, both of the pulp and the skin, opening up great possibilities for the development of novel gourmet products. However, since the introduction of the Désirée variety in 1962, more than 50 varieties have been introduced, principally from Europe [1] [2] . Late blight, caused by the fungus Phytophthora infestans Mont. De Bary, is one of the most important diseases of the crop [3] , and can destroy complete plantations in a short space of time. It is therefore considered to be the most serious problem affecting production worldwide. It affects plants at any stage of their development and early infection can produce losses of up to 100% of production. P. infestans is a very difficult enemy to fight due to its population diversity [4] . Work has been done over many years to obtain resistant cultivars, although in the majority of cases this resistance is overcome within 2 or 3 years, due to the emergence of ever more aggressive isolations. In this context, wild species are used as a source of resistance for potato improvement [5] .
Historically, the first reports of P. infestans in Chile date from the 1950s, supposedly introduced from Argentina, since migrations have played an important role in the dispersion of this fungus. In southern Chile, there have been repeated years with highly aggressive blight attacks on plantations and production losses greater than 50%, with the majority of native varieties and commercial cultivars under cultivation being affected to a greater or lesser degree. The above may be explained by changes in the populations of the fungus towards more aggressive forms, resulting from strains resistant to metalaxyl, mutations, asexual recombinations, incorrect application of fungicides or more favourable environmental conditions [6] . Acuña et al. (2007) [7] determined that the populations of P. infestans in Chile were only of A1 (asexual) mating type, A2 mating types have not been discovered in Chile yet. However, they have experienced a genetic change to genotypes which are highly resistant to metalaxyl and complex pathotypes with up to 9 and 10 avr genes (avirulence genes) (recessive mutation at an Avr locus, not recognized by the corresponding R gene). With respect to the frequency of avr genes of P. infestans in isolations in southern Chile, Acuña et al. (2008) [8] indicate the existence of complex isolations with presence of the majority of the avr genes, with the exception of avr9. During the 2006/2007 and 2007/08 seasons, a greater prevalence was observed of avr11, avr10, avr8, avr7, avr5 avr3 and avr1.
In the Chiloe archipelago, the tubers of native potato varieties have been a staple food of the population for years. Many local varieties and ecotypes might have had high susceptibility to the disease before the arrival of late blight on the island. Nevertheless, since its arrival selection for more resistant clones may have begun, identification studies of some of the avr genes present in isolations recovered from native material show that they are complex, with the presence of various avr genes [8] .
Two types of resistance to the disease may be distinguished: specific and non-specific. These two types of resistance are often called vertical and horizontal by plant breeders. The first is characterised by triggering a hyper-sensitive response in the form of small necrotic lesions, and is called specific, racial, monogenic, qualitative, or complete resistance. It is controlled by major genes (R-genes) of mendelian inheritance, expressed under conditions of dominance. The dominant and recessive alleles of a specific R-gene locus are noted R and r. Genes of this type are incorporated from Solanum demissum and Solanum stoloniferum. This type of resistance is not very long-lasting since P. infestans develops different races of greater virulence which can overcome the resistance [9] . The genes of the pathogen are called avirulence genes (Avr), they are recognized by specific R genes of the host plant in a gene by gene interaction. The only isolations able to attack a plant holding a specific R allele are those with the corresponding recessive non expressed avirulence allele. The dominant and recessive alleles of an Avr locus are noted Avr and avr [10] [11] .
Horizontal resistance, known also as quantitative or field resistance, is governed by a large number of minor genes (r), with small effects and additive in character; it apparently does not involve gene by gene interaction and therefore it is assumed to be uniformly distributed against all the races of the pathogen, delaying their development and thus allowing timely treatment to avoid their propagation. Its stability is attributed to its capacity to maintain a balance between all the races of P. infestans present in a location [12] . Horizontal resistance acts in different ways, either through the production of toxic exudates on the leaf surface, confinement of the fungal structures to the cell walls, low colonization of the mesophyll, slow collapse of the petioles or reduction of the reproductive range of the pathogen; in other words it raises physiological or chemical barriers in the tissues of the host which retard the range and frequency of the penetration and reproduction of the oomycete [13] [14] . Horizontal resistance interrupts or retards one or more stages of the life cycle of the pathogen. In addition, other factors come into play such as certain characteristics of the plant, for example, the thickness of the leaf cuticle and/or the presence of substances which inhibit the development of the pathogen, duration of the latency period, etc. [15] . Plants which have this type of resistance are infected in the field, but the damage and the percentage of the crop infected are much lower than in susceptible plants. Horizontal-non-specific resistances are more stable over time and space compared with specific resistances driven by R-Genes [15] - [19] . When a variety with horizontal resistance is exposed in the field to conditions which are very favourable for the pathogen, and to high inoculum pressure, resistance is lost and the variety behaves like a susceptible variety, however, the resistance is maintained and the variety returns to resistant behaviour in less favourable environments for P. infestans. Zlesak and Thill (2004) [20] indicate that the level of quantitative resistance in the existing germplasm banks of S. tuberosum appears to be insufficient to allow a significant reduction in the use of fungicides in the future. Secor et al. (2009) [21] add that, due to the genetic complexity of the host-pathogen interaction and the plasticity of the genome of this pathogen, stable resistance has been difficult to introduce by traditional means of genetic improvement. With respect to the resistance components, those which are used permanently are: latency period, size of lesion, lesion growth rate, and relative sporulation area. In general, they all show considerable variation between cultivars and localities [9] [14] [22] - [24] . Based on the above, and considering that in Chile the resistance to late blight of native material has not been characterised, the objective of the present study was to evaluate the resistance of 10 accessions to P. infestans, by in-vitro inoculation of inoculation of detached leaflets with a complex isolation of the fungus.
2. Material and Methods
2.1. Plant Material
The plant material evaluated was taken from the Chiloé archipelago (Table 1). These native Solanum are characterised by their possession of a high genetic diversity, and were selected for their good behaviour against late blight observed in the field in previous years. The differential Craig’s Royal (R0) was used as a susceptible control, and as a resistant control the advanced line R8906384 belonging to the potato improvement programme of the Institute of Agricultural Research Institute, which has proved to have excellent resistance in Chile and United States [21] . These were included in all the repetitions.
2.2. Protocol for the in-Vitro Inoculation of Detached Leaflets
Twenty tubers of each accession were planted in 1 lt. pots and cultivated under greenhouse conditions at the Agricultural Research Institute (INIA-Remehue). The test was done using young leaflets which were collected
![]()
Table 1. Native Solanum accessions evaluated.
early in the day, before 10:30 AM. These were taken from fully developed plants at age 8 weeks [23] . The isolate used for inoculation was Pi287, a carrier of 10 avirulence factors identified in the country (avr1, avr2, avr3, avr4, avr5, avr6, avr7, avr8, avr10, avr11) [7] . The leaflets were inoculated following the protocol described by Colón, et al. (2004) [25] . The isolate was cultivated in rye agar for 10 to 12 days at 18˚C. The plates were washed with 10 ml sterile distilled water to collect the sporangia. It was then incubated at 4˚C for 3 hours. The humidity chambers were prepared by placing a paper towel inside the containers and a mesh on top, and sprinkling with 10 ml of sterile distilled water to humidify them. For each inoculation, one leaflet from each accession was selected. Completely developed leaves were selected from the top third of the plant. The leaflets were distributed inside the humidity chambers, including the susceptible and resistant controls. One additional leaflet of the susceptible control was taken for inoculation with water. The humidity chambers were duly identified, indicating the repetition, plant number and internal distribution of each accession. Each plate wash was calibrated with the fungus at a concentration of 2 × 103 zoospores × ml−1 using a haemacytometer. Each leaflet was inoculated with 30 µl of the inoculum, adjusted to the concentration indicated. The leaflets were inoculated in the abaxial zone. The specimens were then incubated in a growing chamber with artificial lighting, using white light, and temperature controlled at 18˚C. After 12 hours the drop of inoculum deposited on the leaflet was dried with filter paper.
2.3. Resistance Components Evaluated
The resistance components were evaluated at 72, 96 and 120 hours after inoculation. The components were latency period, leaf necrosis, size of lesion and sporulation level. All the evaluations were done on the abaxial zone of the leaflet. For percentage of infection success, each leaflet was scored 1 or 0 depending on the development or not of an infection after inoculation. Latency period corresponds to the period of time in hours between inoculation and the appearance of the sporangia. This period allows resistance to be classified and the development of the disease in the different accessions to be compared. The sporulation process was monitored as from 72 hours after inoculation. For leaf necrosis, a percentage scale was used where: 0.1% = corresponds to a small, separated necrotic lesion; 1.0% = represents necrosis in the inoculation area; 5% - 100% = corresponds to the total percentage of the leaflet affected by necrosis [25] . This trait gives a measure relative to the leaflet area. A big leaflet with a big lesion can present the same value as a small leaflet with a smaller lesion. The size of the lesion affected by the pathogen was determined by measuring the length and width of the necrotic area. Assuming an elliptical development of the lesion, its size (SL) was estimated in square centimetres (cm2) by SL = (π*L1*L2)/4, where L1 and L2 correspond to the length and width of the lesion [26] . This trait informs on the absolute size of the necrotic area independently of the area of the leaflet. If all the leaflets had the same area whatever the variety this trait should be highly correlated with the previous one. For level of sporulation, a scale of 1 to 3 was used, where: 1 = represents absence of sporulation, 2 = corresponds to slight to moderate sporulation (50% of necrosed area with sporangia), 3 = corresponds to intense sporulation (100% of necrosed area with sporangia) [25] . The varieties were compared for the proportion of non infected leaflets using a Chi2 test for homogeneity. The data for latency period, leaf necrosis, size of lesion and sporulation level were subjected to an analysis of variance (anova) in accordance with the experiment design. The mean values were compared using Duncan’s statistic (p ≤ 0.05). We estimated the heritabilities of the different components as H2 = VarG/(VarG + VarR) and  = VarG/(VarG + VarR/Nrep) with VarG the genetic variance between accessions, VarR the environmental variance and Nrep the number of repetitions. VarG was estimated as (MSG-MSR)/Nrep, with MSG and MSR the genetic and residual mean squares. As the anovas were performed on the sole leaflets which developed an infection, Nrep was not the same for the different genotypes, consequently we used the harmonic mean among genotypes which value was 32.3 (close to the arithmetic mean 33.1).
= VarG/(VarG + VarR/Nrep) with VarG the genetic variance between accessions, VarR the environmental variance and Nrep the number of repetitions. VarG was estimated as (MSG-MSR)/Nrep, with MSG and MSR the genetic and residual mean squares. As the anovas were performed on the sole leaflets which developed an infection, Nrep was not the same for the different genotypes, consequently we used the harmonic mean among genotypes which value was 32.3 (close to the arithmetic mean 33.1). 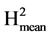 is a measure of the level of repeatability, its value is an estimate of the expected correlation between the genetic means estimated on two independent experiments of the same genotypes repeated Nrep times in each experiment. It is also an estimate of the coefficient of determination (R2) of the genetic value by the mean phenotypic value of the variety. Based on the estimated values of VarG and VarR we also calculated
is a measure of the level of repeatability, its value is an estimate of the expected correlation between the genetic means estimated on two independent experiments of the same genotypes repeated Nrep times in each experiment. It is also an estimate of the coefficient of determination (R2) of the genetic value by the mean phenotypic value of the variety. Based on the estimated values of VarG and VarR we also calculated 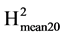 = VarG/(VarG + 2*VarR/Nrep). This coefficient tells us about the repeatability and the coefficient of determination (R2 as previously defined) in an experiment with twenty repetitions. It allows assessing the possibility of reducing the number of repetitions per experiment in order to increase the number of varieties tested.
= VarG/(VarG + 2*VarR/Nrep). This coefficient tells us about the repeatability and the coefficient of determination (R2 as previously defined) in an experiment with twenty repetitions. It allows assessing the possibility of reducing the number of repetitions per experiment in order to increase the number of varieties tested.
3. Result and Discussion
When leaflets of 10 accessions of native potato Solanum, an advanced line and the cultivar Craig’s Royal (R0) were inoculated with zoospores from isolation Pi 287, clear differences were observed in the development of the disease. Table 2 shows the proportions of infected and uninfected leaflets for each variety. These differences were highly significant (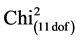 = 42.81, P value = 1.2 × 10−5). The highest contributions to the Chi2 were those of R-8906384 and UCT-34Cor (14.42 each) both presented 40% of non infected leaflets (16 non infected vs 24 infected). UCT-18Mn and UCT-9MgM, which presented 95% of leaflets with sporulation (38 infected vs 2 non infected) were also important contributors to the Chi2 (4.23 each). Other genotypes had a low contribution to the Chi2. This test revealed three groups (Table 4), one group with the lower probability of infection success (24 infected out of 40), one group with a very high probability of infection success (38 infected out of 40) and a group with medium to high probability of infection success (31 to 36 out of 40, equivalent to 77.5% to 90%). This group included the susceptible control Craig’s Royal and the accession UCT-35AzC, both with 36 infected.
= 42.81, P value = 1.2 × 10−5). The highest contributions to the Chi2 were those of R-8906384 and UCT-34Cor (14.42 each) both presented 40% of non infected leaflets (16 non infected vs 24 infected). UCT-18Mn and UCT-9MgM, which presented 95% of leaflets with sporulation (38 infected vs 2 non infected) were also important contributors to the Chi2 (4.23 each). Other genotypes had a low contribution to the Chi2. This test revealed three groups (Table 4), one group with the lower probability of infection success (24 infected out of 40), one group with a very high probability of infection success (38 infected out of 40) and a group with medium to high probability of infection success (31 to 36 out of 40, equivalent to 77.5% to 90%). This group included the susceptible control Craig’s Royal and the accession UCT-35AzC, both with 36 infected.
In the following, the evaluations of the different resistance components have been carried out on the infected leaflets only. In this way it was possible to compare the behaviours of the varieties once the infection was established. No significant differences were found for any trait measured 72 hours after infection. For all the traits 96 h and 120 h after inoculation we can note a general trend toward low or very low broad sense heritabilties (Table 3). This is consistent with the results of a previous experiment (data not shown) whose results were unusable because the number of repetitions were too limited. It was these preliminary results that have led us here to greatly increase the number of repetitions. For latency period, no significant differences were found among varieties for this trait, once a leaflet was infected the pathogen underwent its cycle at the same pace whatever the genotype. The mean duration between the inoculation and the appearance of the first sporangia was about 4 days. The results for leaf necrosis show highly significant differences among the accessions in the evaluations at 96 (Necr-96h) and 120 hours (Necr-120h) after inoculation (Table 3 and Table 4). The differences in the presence of leaf necrosis became apparent from 96 hours, reaching their maximum expression at 120 hours after inoculation. Nevertheless the anova showed a slightly better discrimination power at 96 h after inoculation, the F values for Necr-96h and Necr-120h were 7.81 and 5.38 respectively. The heritabilities of the mean values (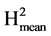 ) were very high and a reduction of the number of repetitions from 40 to 20 would be quite possible for these traits. UCT-15MgRo expressed the worst behaviour for Necr-96h, followed by UCT-34Cor and UCT-6Gc. The resistant and the susceptible controls were scored equivalent 96 and 120 hours after inoculation. Interestingly the susceptible control “Craig’s royal” exhibited the best value for Necr-120h. UCT-15MgRo remained the worst 120 hours after inoculation but UCT-34Cor exhibited an average value. This might indicate a late onset of a resistance process which contributed to the slowdown of the progression of the disease. Four native varieties (UCT-26Ach, UCT-3CI, UCT-1Ma and UCT-30Ño) behaved quite well 120h after inoculation. The sizes of
) were very high and a reduction of the number of repetitions from 40 to 20 would be quite possible for these traits. UCT-15MgRo expressed the worst behaviour for Necr-96h, followed by UCT-34Cor and UCT-6Gc. The resistant and the susceptible controls were scored equivalent 96 and 120 hours after inoculation. Interestingly the susceptible control “Craig’s royal” exhibited the best value for Necr-120h. UCT-15MgRo remained the worst 120 hours after inoculation but UCT-34Cor exhibited an average value. This might indicate a late onset of a resistance process which contributed to the slowdown of the progression of the disease. Four native varieties (UCT-26Ach, UCT-3CI, UCT-1Ma and UCT-30Ño) behaved quite well 120h after inoculation. The sizes of
![]()
Table 2. Percentage of infected leaflets (120 hours after inoculation).
![]()
Table 3. Global statistics based on the anovas.
H2: broad sense heritability = VarG/(VarG + VarR).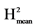 : VarG/(VarG + VarR/Nrep).
: VarG/(VarG + VarR/Nrep). : VarG/(VarG + 2*VarR/Nrep).
: VarG/(VarG + 2*VarR/Nrep).
![]()
Table 4. Comparison of means for the different traits.
(*) The grouping for % infection is based on the Chi2 test. (**) Duncan grouping at the 5% level, varieties with the same letter are not statistically different. (#) Duncan grouping at the 10% level, varieties with the same letter are not statistically different.
lesion show significant differences among the potato accessions in the evaluations at 96 (Cm2-96h) and 120 hours (Cm2-120h) after inoculation. The differences in the size of the lesions became clearly apparent from 96 hours, reaching their greatest size at 120 hours after inoculation (Table 3 and Table 4). The measure after 120 h appeared the most discriminant between genotypes (higher F value and higher heritabilty). The heritability of the means 120 h after inoculation was high enough to consider a reduction to 20 repetitions of the experimental design. Some of the native varieties (UCT-3CI, UCT-1Ma and UCT-34Cor) showed a good behaviour, as good as the resistant control. The susceptible control Craig’s Royal exhibited the worst behavior for this trait at the two period of scoring.
The levels of sporulation show significant differences among accessions in the evaluations at 96 h (Spor-96h) and 120 h (Spor-120h) after inoculation (Table 3). Although significant, differences revealed by the test at 96 hours were not very discriminating. For Spor-96h the F test was significant at the 2% level only and the Duncan multiple range test at the 5% level ranked all varieties in a single group. Concerning Spor-120h it appeared very discriminatory. This shows that sporulation was not yet sufficiently developed 96 hours after inoculation. The F statistic for Spor-120h was the highest of all those observed on all the traits. Consequently Spor-120h also exhibited the highest heritabilities and a reduction in the number of repetitions would be possible while maintaining a strong discriminatory power for this trait. Four accessions (UCT-3CI, UCT-1Ma, UCT-6Gc and UCT-9MgM) expressed the higher levels of sporulation. Two native accessions (UCT-34Cor, UCT-26Ach) and the susceptible control “Craig’s Royal” expressed the lower levels.
All notations made 72 hours after inoculation showed no significant difference, we can conclude that it is possible to make the economies of these notations. Scoring the percentage of necrosis was found more discriminating at 96 compared to 120 hours after inoculation. The reason was that it was more difficult to differentiate the varieties for which the percentage of necrosis was very high, the mean values among all varieties for Necr- 96h and Necr-120h were 31% and 61% respectively. For this character scoring four days after inoculation was optimal when the necrosis occupied about 50% of the leaflet of the more susceptible varieties. Nevertheless, the observed rankings were almost the same from one scoring to another. For the sizes of the lesions the later scoring was more discriminatory but the rankings were broadly equivalent between the two notations. The levels of sporulation were much more discriminatory between varieties only 120 h after inoculation.
Inoculation with a complex isolate of a fungus which carries 10 avr genes identified in the country demonstrates the presence of a wide range of expression of partial resistance to late blight, coinciding with the findings reported by Micheletto et al., (2000) [15] and Barquero et al. (2005) [23] . The resistant control R-8906384 showed an excellent behavior for each component of the resistance. He is always in the best group for each measurement. It is even consistently classified as first for the various measurements made 96 hours after inoculation. No native accession so well behaved, however UCT-34Cor ranked consistently among the very best regardless of the measured component except for Necr96h. UCT-26Ach performed also quite well for the different components except for the size of the lesion 120 h after inoculation. No accession was found the worst for all the components, for example UCT-3CI and UCT-1Ma which were among the worst for the level of sporulation behaved very well for the size of the lesion. Even the variety described as susceptible showed good performance for some traits, such as leaf necrosis or the level of sporulation. These results suggest that resistance factors are present in different varieties, even those considered susceptible. It is only the combination of these different components that leads to a good level of resistance. In accordance to the results of Colon et al. (1995) [14] which consider that the most important resistance components in Solanum tuberosum are efficiency of infection (equivalent to the probability of infection success), growth rate of the lesion and sporulation capacity, R- 8906384 the resistant control and UCT-34Cor which had the best performance in the field trials revealed the highest performance for these three components. This accession achieved one of the highest resistance to the disease under field conditions and showed one of the lowest values AUDPCr (AUDPCr: 0.05) [27] . We also found a positive correlation between the probability of in vitro infection and the AUDPCr measured in the field. A lower correlation (only significant at the 10% level) was found between the AUDPCr and the level of in vitro sporulation and that for both measurements made 96 h or 120 h after inoculation. Results from Flier et al. (2003) [9] show that the sporulation density and the growth rate of the lesion are not correlated with the leaf resistance measured by the AUDPC. Barquero et al. (2005) [23] report low correlation between the field AUDPC data and evaluations of detached leaflets in the laboratory. The possible causes of this low correlation are the influence exercised by environmental and genetic factors which determine resistance and the impossibility of finding an individual offering all the genetic factors which determine the virulence of the pathogen [22] .
In the present study, although resistance factors were present in different varieties and accumulated in some more than others, we did not observe completely immune varieties. In the last decade the populations of P. infestans in southern Chile have undergone a genetic change [7] , which is related to the severity of the attacks in recent seasons. Changes in aggressiveness during the course of an epidemic suggest selection for more aggressive isolations. This selection may affect the durability of partial resistance and may explain the progressive reduction in the partial resistance of the materials. Although only the asexual phase of P. infestans is known in Chile, it is possible that specificity for stability to late blight may exist in the Chilean germplasm, stimulated by genetic modification of the populations or immigration of new pathotypes. The resistance observed in the Solanum accessions is probably due to the action of menor genes, since the isolate Pi287 is carrier of all the avr factors identified in Chile (avr1, avr2, avr3, avr4, avr5, avr6, avr7, avr8, avr10, avr11), a situation which would stimulate the expression of real field resistance, uniformly distributed against all the races of the pathogen, emphasising the differences between the varieties when they grow in the same environment in Chiloe. It is also the case that the use of fungicides to control the disease is not usual in
Chiloe
Island
. The above may explain in part the frequent strategy of establishing the crop with a mixture of varieties for protection against attacks of late blight.
4. Conclusions
The different resistance components demonstrated that within the material of Chilean native Solanum, there was a wide range of variation to late blight. The resistant control (R-8906384) and the accessions UCT-34-Cor presented the lowest infection efficiency, small sizes of the lesions and low levels of sporulation classifying them as the most resistant. This suggests low efficiency of the infection and high resistance to penetration in these materials. The more susceptible accessions often presented a good level of resistance for one or few components. It would be of great interest to go into the genetic control of the different resistance components to know if the same level of resistance expressed for a component by different accessions is under the same genetic control or not. Deciphering the genetic architecture of the non specific resistance is a challenge for the development of long lasting resistant varieties.
It is important to continue evaluating resistance to late blight in native potato material. This is a practical and economic method of combating the disease, especially through non specific resistance, since it is a more stable and durable alternative against the various variants or races of the pathogen. The study presented here allows proposing less onerous in vitro protocols in order to test more genotypes for the same level of experimental investment.
Acknowledgements
We thank Florence Esnault, UMR 1349 IGEPP INRA, Ploudaniel, France, for their comments on this work.