Influence of Geographical Regions on Catechin and Caffeine Levels in Tea (Camellia sinensis) ()
Received 17 February 2016; accepted 20 March 2016; published 23 March 2016

1. Introduction
Tea is manufactured from the young tender leaves of the plant Camellia sinensis. The tea plants grow mainly in warm (tropical and subtropical) climates and can thrive at an altitude range of 1500 - 2700 m above mean sea levels and require at least 1270 mm of rainfall a year and prefer acidic soils [1] . Since its introduction, tea cultivation in Kenya has expanded tremendously in volume and in the tea growing regions mainly the East (Kiambu, Thika, Muranga, Meru, Nyeri, Kirinyaga and Embu) and West (Kericho, Kisii, Nyamira Bomet, and Nandi) of the Rift valley. Additionally, cultivation of the crop has expanded to include even marginal areas initially considered unsuitable for the crop [2] . There exist small to large differences in climatic and environmental conditions between the different growing regions, which consequently affects yield and quality of the resulting tea products.
Catechins and caffeine constitute the most important quality parameters in tea reference. Catechins are among the major polyphenolic compounds in the tea plant constituting up to 30% of the dry weight in a freshly picked tea leaf [3] . Catechins have been reported to be potent biomolecules responsible for the health benefits associated with regular drinking of tea [4] [5] . Studies have shown that catechins possess beneficial physiological activities such as antioxidants [6] [7] , hypocholesterolemiant [8] [9] , anti-atherosclerotic [10] , anti-inflammatory [11] , and anti-HIV activities [12] .
Caffeine is highly valued for its central nervous stimulating properties [13] . It is associated with improved performance of tasks that require sustained effort and attention [14] , speed of response [15] , feelings of well- being and improved moods [16] . Several factors have been shown to be responsible for the tea quantity and ultimately the amount of biomolecules that are synthesized and accumulated by the plant [17] . Such factors are attributed to the environment such as climate [18] and altitude [19] of the growing region, agronomic factors such as farm management, harvesting practices and fertilizer applications and processing procedures [2] . Agronomic factors are important but considered controllable with previous studies by [20] [21] suggesting the adoption of different optimized management techniques such as fertilizer rates used, post harvesting techniques and plucking intervals that suit the different growing regions. Environmental factors have been shown to affect the levels of biomolecules synthesized and consequently the quality of the final tea product by influencing the rates of shoot growth [21] - [23] . Altitude differences affect the rates of shoot growth in tea plants, with a high altitude slowing the rate consequently increasing synthesis of biomolecules while low altitudes increase the rate of shoot growth causing a decline in synthesis of the biomolecules. Mean temperature and rainfall intensities between growing regions play an equally important role in influencing tea quality with high intensities stimulating a fast shoot growth compared to low intensities which stimulate a slower rate leading to accumulation of large amounts of the biomolecules [24] [25] . However, the extent of variations in synthesized biomolecules is depended on the clonal genotype as individual clones are unique on their levels of synthesized biomolecules. Therefore some clones are more stable and therefore less susceptible to changes in composition of biomolecules due to differences in environmental conditions [2] [22] . Despite these observations, research work detailing the extent of influence of the underlying environmental conditions on the clones grown in both the Timbilil and Kangaita regions is not well described. As the tea industry seeks to produce high quality and diversified products to expand its market outlets and satisfy different consumer needs, the need to study TRI’s two main tea growing stations is highly necessary. In this study, levels of catechins and caffeine in clones grown in both sites were carried out to establish how quality parameters are influenced.
2. Methodology
2.1. Description of Study Sites
The study sites included Timbilil Estate (latitude 0˚26'S, longitude 37˚1̍̕5'E, altitude 2020 m amsl) in the West of the Great Rift Valley and Kangaita substation (latitude 0˚30'S, longitude 37˚16'E, altitude 2180 m amsl) in the East of the Great Rift Valley. Kangaita experiences a weakly bimodal rainfall distribution of 2040 mm annually with peaks in April/May and October/November, while Timbilil has rains most of the year, except in the months of December, January and February when there is drought with an annual rainfall of 2175 mm. Kangaita has a mean annual temperature of 15.5˚C while Timbilil has a mean annual temperature of 16.6˚C.
2.2. Tea Samples
About 300 g of fresh green tea leaf samples (consisting of the two leaves and a bud) were obtained in triplicates from each of 60 tea cultivars grown in both study sites; Timbilil Estate and Kangaita substation of the TRI. These clones were selected from pre existing ongoing experimental trials in the two sites.
2.3. Sample Processing
The samples were first steamed for one to two minutes then dried in a micro-wave oven. They were then pulverized with a grinder into fine powder used in assaying for catechins and caffeine. The analysis was done using the Tea Research Institute established and published wet chemistry procedures High Performance Liquid Chromatography (HPLC) and spectrophotometric protocols.
2.4. Determination of Catechin Levels and Profiles
Approximately 0.2 ± 0.001 g of each of the sample was weighed into an extraction tube. Five milliliter of 70% methanol at 70˚C was dispensed into the sample as an extraction mixture and vortexed until properly mixed. Heating of the extraction tube was continued in the water bath at 70˚C for 10 min with mixing in the vortex mixer (VM-1000, Taiwan) after every 5 min. The extraction tubes were then removed from the water bath (Digital water bath LWB-122D, Korea) and allowed to cool at room temperature. The tubes were then placed in a centrifuge (HSCEN-204, MRC) at 3500 rpm for 10 minutes and the supernatant decanted into a graduated tube and the extraction procedure repeated. Both extracts were combined and the volume made up to 10 ml with cold 70% methanol. One milliliter of the sample extract was transferred into a graduated tube and diluted to 5 ml with a stabilizing solution (10% v/v acetonitrile with 500 μg/ml Ethylenediaminetetraacetic acid (EDTA) and ascorbic acid). The solution was filtered through a 0.45 μm nylon membrane filter and a 20 μl aliquot of this solution was injected into HPLC for analysis.
A high performance liquid chromatography method [26] was used to assay for the tea catechins. A Shimadzu LC 20 AT HPLC system fitted with a SIL 20A auto sampler and a SPD-20 UV visible detector with a class LC 10 chromatography workstation was used for analysis of the prepared samples. A Gemini 5 µM C6-Phenyl, 250 mm × 4.6 mm (Phenomenex, Torrance, CA, USA) separation column with a Reodyne precolumn filter disk was used. The sample was degassed before injection into the HPLC system. A gradient elution was carried out using the following solvent systems: Mobile phase A (acetonitrile/acetic acid/double distilled water-9/2/89 v/v/v) and mobile phase B (acetonitrile/acetic acid/double distilled water-80/2/18 v/v/v). The mobile phase composition for a binary gradient condition was started at 100% mobile phase A for 10 minutes then over 15 minutes a linear gradient to 60% mobile phase A, 32% mobile phase B and held at this composition for 7 minutes. The condition was reset to 100% mobile phase A and then allowed to equilibrate for 10 minutes before the next injection. The flow rate of the mobile phase was kept at 1 ml/min and the temperature in the column maintained at 35˚C ± 0.5˚C throughout the analyses. Identification of individual catechins was carried out by comparing the retention times and UV-absorbance of unknown peaks with peaks obtained from the mixed known catechin standards under the same conditions. The quantification of catechins was performed at 278 nm and was achieved using a caffeine standard with a calibration curve R2 = 0.9984 in conjunction with the consensus individual catechin Relative Response Factor (RRF) values with respect to caffeine calculated on a dry matter basis. The total catechin content as a percentage by mass on a sample dry matter basis was given on the summation of individual catechin contents.
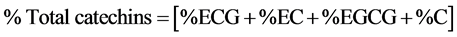
Since caffeine is used as a standard in identification of catechins, its levels were quantified alongside those of individual catechins by comparing the samples’ retention times with that of the caffeine standard.
2.5. Data Analysis
All determinations for the levels of caffeine, total and individual catechin fractions, were done in triplicates and the data subjected to analysis of variance using GENSTAT-C statistical software packages. The least significant differences Test (LSD) was used to separate the means.
3. Results and Discussion
Results shown in Table 1 revealed significant differences in the levels of catechins and caffeine between Kangaita and Timbilil sites.
3.1. Total Catechins
Kangaita clones had significantly higher (P < 0.05) total catechin content with a mean value of 18.7% compared to 16.2% observed for Timbilil clones. However, 10 clones namely; BBK 35, TRFK K-Purple, TRFK 301/5, TRFK 430/90, TRFK 91/1, AHP SC 31/37, TRIT 201/16, TRIT 201/44, TRIT 201/47 and TRIT 201/82 from Timbilil performed better than Kangaita clones.
3.2. Individual Catechins Fractions
3.2.1. Epigallocatechin Gallate
Clones from Kangaita region had significantly high EGCG values with a mean value of 7.9% compared to 6.7% observed in the clones from Timbilil. However, BBK 35, TRFK K-Purple, TRFK 400/10, TRFK 430/90, AHP SC 31/37, TRIT 201/16, TRIT 201/44, TRIT 201/47, TRIT 201/50, TRIT 201/55, TRIT 201/70, TRIT 201/73, TRIT 201/75 and TRIT 201/82 from Timbilil had higher EGCG levels.
3.2.2. Epicatechin Gallate
Results obtained showed that Kangaita clones had significantly high ECG levels (mean value of 3.3%) than Timbilil clones (mean value of 2.5%) with exceptions of the clones BBK 35, AHP SC 31/37, TRIT 201/16, TRIT 201/44, TRIT 201/50, TRIT 201/73 and TRIT 201/82.
3.2.3. Epigallocatechin
Kangaita clones had a significantly high mean value of EGC (5.2%) compared to Timbilil clones (5%). However clones EPK H 81/22, TRFK 31/8, TRFK 12/12, KTDA KAG 4, AHP F7/346, MICHI (51/10/20), TRFCA SFS 150, STC M3, BBK 5, TRFK 303/577+, GW EJULU-L, BBK 35, BBK 21, AHP SC 12/28, TRFK K PURPLE, TRFK 301/1, TRFK 301/3, TRFK 301/4, TRFK 301/5, TRFK 301/6, TRFK ST 543, TRFK 306/1, TRFK 306/3, TRFK 91/1, AHP SC 31/37, TRIT 201/47 and TRIT 201/82 from the Timbilil site performed better.
3.2.4. Epicatechin
Significant difference was observed for EC levels with Kangaita clones having a high mean value of 1.5% compared to 1.4% in the Timbilil clones. However, clones KTDA KAG 4, AHP F7/346, MICHI (51/10/20), TRFCA SFS 150, STC M3, BBK 5, TRFK 303/577+, BBK 35, TRFK 301/1, TRFK 301/3, TRFK 301/4, TRFK 301/5, TRFK 301/6, TRFK 306/1 and TRIT 201/47 in Timbilil performed better.
3.2.5. Catechin (+C)
Results for the variations in simple catechins between the two regions among the studied clones showed significantly high levels in Kangaita (mean value 0.7%) compared to those in Timbilil which had a mean value of 0.6% with clones KTDA KAG 4, STC M3, TRFK 301/3, TRFK 306/1, TRFK 306/4, TRFK 91/1, AHP SC 31/37 and AHP CG28V929 performing better in Timbilil site.
3.2.6. Caffeine
Caffeine analysis results for the two regions showed an interesting pattern, different from that observed for individual catechins. A significant difference was observed with Timbilil clones containing high caffeine levels (mean value of 4.2%) compared to Kangaita clones (mean value of 3.8%) as depicted in the Table 1. However, clones EPK H 81/22, EPK C182/40, AHP F5/222, AHP F7/346, MICHI (51/10/20), AHP MNI 1/96, TRFCA SFS 150, STC M3, BBK 5, TRFK 303/577+, TRFK 54/40, BBK 21, AHP SC 12/28, TRFK-K PURPLE, TRFK
![]()
![]()
Table 1. Differences in the contents (%) of catechins and caffeine between Kangaita (K) and Timbilil (T) study sites.
EGC-Epigallocatechin, C-Catechin, CAFF-Caffeine, EC-Epicatechin, EGC-Epigallocatechin gallate, ECG-Epicatechin gallate, TC-Total catechins.
301/6, TRFK 400/1, AHP PC 81, AHP SF 186, AHP CG28V929 and TRIT 201/43 in Kangaita performed better.
There were significant interactions (p ≤ 0.05) between the growing region and the clones, an indication that synthesized catechins and caffeine differ in regions. This is because while clonal performance is primarily genetically linked, the growing region and the underlying conditions have some level of influence on the synthesis of the biomolecules as evidenced in this study where the studied clones performed differently in the two study sites. This implies that the two factors, region and clone should be taken into consideration during tea production to ensure that high quality of the final product is achieved. From the total catechins results, several clones showed wide variations between the two regions such as EPK C12 with 22.2% in Kangaita and 16.5% in Timbilil, TRFK 100/5 with 23.9% in Kangaita and 16.7% in Timbilil. The clones in Kangaita therefore provide the suitable raw materials for production of diversified catechins-rich products such as green teas and catechin extracts. Apart from being the most biologically active biomolecules in green tea, catechins are also important precursors of black tea theaflavins and thearubigins and are therefore important quality markers. These observations of wide variations in catechins between the two regions imply that clones must be thoroughly evaluated for their performance in the intended cultivation area to test their suitability before their release. However, in clones such as TRIT 201/55, the variation in the two regions was not statistically different with 20.0% in Kangaita and 19.8% in Timbilil, an observation consistent with that of a study by Wachira et al., (2002) suggesting that irrespective of the region of production, some tea genotypes exhibit resistance to yield variations and thus quality. This observation implies that such clones with least variation between the two growing regions are more stable and thus will have little variations when grown in other tea growing regions of different climatic conditions such as Sotik, Maragwa and Embu. Utilization of such clones with adaptations to varied environmental conditions will ensure increased and consistent production of tea with uniform biomolecule composition and therefore produce teas of uniform quality. This observation is similar to a recent study [27] which found significant differences in chemical quality although based on the micronutrient level between teas from East and West of the Great Rift Valley. It is clear from the results that most of the clones in Kangaita perform better compared to those in Kericho based on their total and individual catechins. From these results, the influence of regional differences and their underlying climatic conditions on the differential synthesis and accumulation of these bioactive compounds is responsible for quality in tea. This is in agreement with previous studies [18] [21] - [23] [28] whereby factors such as seasonal climate, altitude, temperature and total rainfall density have been shown to affect the tea’s yield, overall growth rate and synthesis and accumulation of the chemical compounds responsible for the quality characteristics.
Altitude has an influence on the growth of the tea plant whereby shoots of teas grown in high altitude regions grow and mature slowly and in the process accumulate high amounts of these polyphenolic catechins (quality markers) and develop a richer flavor [18] [29] [30] . This agreed with the present study in caffeine composition. However, this study differs with such observations on catechins levels in that Kangaita has a relatively lower altitude (2020 m) than Kericho (2180 m) yet the tea clones performed better implying that other factors could be contributing to the observed differences. Temperature could have contributed to this observation since Kangaita is located near Mt. Kenya which is relatively cooler throughout the year with a mean annual temperature of 15.5˚C compared to 16.6˚C in Timbilil. This environment induces a form of dormancy in the rate of shoot growth consequently increasing the accumulation of catechins in these clones. High temperatures on the other hand stimulate a fast shoot growth but with low levels of these biomolecules [18] . Similar results were observed in previous studies [31] which reported that elevated temperature reduced flavonoid accumulation and inhibited the gene expression of the enzymes in phenylpropanoid and flavonoid biosynthetic pathway; chalcone synthase (CHS), flavanone 3-hydroxylase (F3H) and dihydroflavonol 4-reductase (DFR) in grape berries.
Differences in the amounts of rainfall received in the two regions could have played a role in the variation of catechin composition. Kangaita has a mean annual rainfall of 2040 mm with peaks in the months of April/May and October/November while Timbilil receives 2175 mm of rainfall spread through the year except in the dry season (December-February). A high precipitation like in Timbilil stimulates a fast shoot growth rate resulting in high yields but producing low quality teas compared to the relatively low precipitation rate in Kangaita, an observation supporting an earlier study [25] . It is worth noting that whereas a slow rate of shoot growth contributes to a high synthesis and accumulation of catechins, the mean productivity decreases owing to the fact that development of pluckable shoots takes much longer thus reducing plucking intensity [24] [29] [32] . It’s important to note that clonal genetic differences also affects their individual ability to absorb nutrients even under similar agronomic practices and this consequently affects their ability to synthesize and accumulate these bioactive molecules such as catechins [33] [34] . This explains why some clones in Kangaita had relatively low total and individual catechin content compared to the same clones grown in Timbilil even when the conditions in Kangaita seemingly impacted positively on the catechin levels in majority of the clones.
Caffeine analysis showed that growing conditions in Timbilil favored the synthesis and accumulation of caffeine more than Kangaita. A typical example in the variation was observed in clones such as BBK 35 with caffeine levels of 3.3% in Kangaita and 5.6% in Timbilil and clone TRFK 400/10 with 3.3% in Kangaita and 5.28% in Timbilil. Like catechins, synthesis and accumulation of caffeine has been shown to be affected by climatic factors [35] . Previous studies [36] [37] have revealed that a high relative humidity stimulates caffeine synthesis in tea plants, observations supported by this study. Timbilil has a relatively high annual rainfall (2175 mm) compared to 2040 mm in Kangaita. Consequently, the high mean temperature in Timbilil (16.6˚C) seems to increase synthesis of caffeine in the tea clones compared to those in Kangaita with relatively low mean temperature (15.5˚C). This can be explained by the fact that caffeine synthesis by plants is usually in response to adverse climatic conditions such as the relatively high temperature in Timbilil. The high caffeine levels in clones grown in Timbilil could also be attributed to the high altitude of 2180 m compared to the relatively low altitude of 2020 m in Kangaita. This observation on the influence of altitude on caffeine synthesis concurs with a study by Owuor et al., (1990a) in which they found that a high altitude significantly influenced caffeine synthesis in tea plants by slowing down the rates of shoot growth consequently increasing their accumulation.
The differences in the climatic conditions (rainfall intensity and mean annual temperature) between the two regions are not so wide but significant differences in the levels of catechins and caffeine were observed. This observation corroborates a previous study [2] and refutes earlier assumptions stating that larger climatic differences were necessary for significant quality differences to be observed and that a superior clone selected in one location maintains its desirable attributes within the country.
4. Conclusion
The obtained results imply that a high performing clone in one region should be checked for its stability in new regions to ascertain if it maintains relatively high levels of the desired biomolecules. This is desirable since it could lead to development of region-specific clones. Additionally, Kangaita region, favoring synthesis of relatively high quantities of most biomolecules should be considered for growing teas for manufacture of high quality teas or for extraction of biomolecules for use in pharmaceutical and cosmetic industries.
Acknowledgements
This study was supported by the Tea Research Institute (TRI) of Kenya and published with permission from the Director.
NOTES
![]()
*Corresponding author.