Validated UPLC/Q-TOF-MS Method for Determination of Poliumoside in Rat Plasma and Its Application to Pharmacokinetic Study ()
Received 27 January 2016; accepted 12 March 2016; published 15 March 2016

1. Introduction
Callicarpa kwangtungensis Chun (Verbenaceae, CK), a traditional Chinese medicine, possesses various biological activities such as hemostasis and antioxidation [1] . The herbal preparations of “Kang Gong Yan Tablets” and “Kang Gong Yan Capsules” were made up of extracts from CK (the major constituent). As Chinese patent drugs, they have been used in clinic for nearly 20 years to treat gynecological diseases [2] .
Poliumoside is the major phenylethanoid glycosides (PhGs) abundantly found in CK [3] and many other plants, such as Cistanche sinensis G. Beck [4] , Buddleja officinalis Maxim [5] and Brandisia hancei Hook. F [6] . It is also the chemical maker for both CK and the mentioned herbal preparations on the basis of Pharmacopoeia of the People’s Republic of China 2015. Poliumoside also exhibits various activities including antioxidation [7] , hemostasis [8] , neuroprotection [9] and cell aggregation inhibition [10] .
As the potential pharmacological effects, it is necessary to explore the individual pharmacokinetic characteristics of poliumoside for clinical application. Sun et al. developed an ultra-performance liquid chromatography tandem mass spectrometry (UPLC-MS/MS) method for simultaneous determination of crude extract from CK in rat plasma after oral administration [11] . Nevertheless, the multicomponent in herb may impact their individual pharmacokinetic characteristics [12] . The pharmacokinetic behavior of poliumoside after administration of its single form may be different compared with the crude extract from CK. On the other hand, the intravenous administration in rats should also be taken into consideration to confirm the absolute bioavailability of poliumoside. Accordingly, a systematic research on the pharmacokinetics of poliumoside needs to be conducted imminently.
The aim of this article was to develop a rapid and selective UPLC/Q-TOF-MS method for the determination of poliumoside in rat plasma after oral and intravenous administration. This method combines the capabilities of scanning (quadrupole) and high resolution (time of flight). And so far as we know, it has never been used for the analysis of poliumoside in plasma samples. Furthermore, the detailed pharmacokinetic parameters and bioavailability were present for the further research of poliumoside.
2. Experimental
2.1. Chemical and Regents
Poliumoside (Figure 1, purity > 98%) and caffeic acid (used as internal standard (IS) were purchased from Chengdu Herbpurity Co. Ltd. (Chengdu, China). Analytical grade trichloroacetic acid (TCA) was purchased from Tianjin Chemical Corporation (Tianjin China). Acetonitrile, methanol and formic acid were all of HPLC grade.
![]()
Figure 1. The chemical structure of poliumoside and caffeic acid.
2.2. Instruments and Conditions
Chromatographic separation of the analyte was performed on Acquity UPLC system with BEHC18 column (2.1 × 100 mm, 1.7 μm, Waters, Milford, MA, USA). An aliquot of the sample (5 μL) was injected into the UPLC system for analysis. Gradient elution was performed with formic acid (0.1%) in water (mobile phase A) and acetonitrile (mobile phase B) at a flow rate of 0.4 mL/min. The gradient elution process was used as follows: 5% B at 0 to 1 min, 5 to 95% B at 1 to 3 min, then back to the initial condition within 1 min. Before the next injection, all the samples would undergo a needle wash program to minimize the pollution, the solvent for needle wash containing methanol and water (v/v, 50:50). The column temperature was maintained at 45˚C.
Mass spectrometry was performed on the Xevo G2 Q-TOF MS (Waters) equipped with the electronic spray ion (ESI) [13] under negative ion mode. The extraction cone, sampling cone and capillary voltages were 4, 40 and 3000 V, respectively. Nitrogen used as cone and desolvation gas were set to 30 and 800 L/h, respectively. The temperature of source and desolvation were 100˚C and 250˚C, respectively. Mass spectra were recorded in the range of m/z 50 - 1000. An external reference 2 μg/mL leucine enkephalin (m/z 556.2771) was infused at 5 μL/min to guarantee mass accuracy of the entire assay. The ratio mass-to-charge of poliumoside and IS were 769.2537 and 179.0328 respectively. MS quantitation for pharmacokinetics was performed on the full scan analysis and extracted ion chromatograms using MassLynx version 4.1.
2.3. Calibration Standards and Quality Control Samples
Stock solutions of poliumoside (1 mg/mL) were prepared in saline. Working solutions for control and calibration samples were generated by gradient dilution of the stock solution with methanol:water (60:40). The 10% TCA used as precipitating agent was prepared in purified water. The IS (caffeic acid) was prepared by dilution of the stock solution (100 μg/mL) with the same solvent to a concentration of 10 μg/mL. All of the described solutions were stored at 4˚C before use.
The calibration solutions were obtained by spiking appropriate volume of working solutions into the aliquots of 100 μL blank rat plasma, and the plots were set up at the range of 50 - 10,000 ng/mL (50, 100, 625, 1250, 2500, 5000, 10,000 ng/mL). As the same to calibration standard, quality control (QC) samples were prepared in three different plasma concentration level (200, 1000, 8000 ng/mL). All of the samples were stored at −20˚C.
2.4. Sample Preparation
The rat plasma samples, calibration standard solution and the QC samples were thawed at room temperature before use and put them on the ice for the consideration of stability. Plasma samples were extracted through protein precipitation method by 10% TCA. In brief, an aliquot of 20 μL IS working solution (10 μg/mL) was added into 100 μL plasma samples in a 1.5 mL polypropylenes tube, followed by vortexing for 30 seconds. Subsequently, 80 μL of 10% TCA was added into each tube, vortexing for another 3 min. The mixture was centrifuged under 13,000 g for 15 min at 4˚C. The supernatant was collected and transferred into a new tube. Then, 5 μL of the supernatant was injected into the UPLC/Q-TOF-MS system for the further analysis.
2.5. Method Validation
2.5.1. Selectivity
The selectivity was verified through the comparison of the chromatograms of blank plasma, blank plasma spiked with poliumoside and caffeic acid, and rat plasma testing samples. To investigate the specificity and make the result dependable, three different batches were tested following the same procedure.
2.5.2. Linearity and Lower Limit of Quantification (LLOQ)
To construct the calibration curve, the spiked calibration samples in gradient were analyzed in three consecutive days. Peak area ratios of poliumoside to IS were plotted against analyte concentrations and the date was well fitted by least-square regression using 1/x2 as the weighting factor in the concentration range of 50 - 10,000 ng/mL.
2.5.3. Accuracy and Precision
The inter-day accuracy and precision were assessed by analyzing six replicated QC samples at three concentration levels (low, middle and high level) on three separated days. The intra-day accuracy and precision were assessed by analyzing the samples on the same day. Concentrations were calculated with calibration curves obtained daily. The relative error (RE) and relative standard deviation (RSD) were used to evaluate the accuracy and precision, respectively.
2.5.4. The Recovery and Matrix Effect
The recovery of poliumoside was measured by comparing the peak area of QC samples with those of reference QC solutions reconstituted in blank plasma extracts at low, middle and high level (n = 6). The matrix effect was evaluated by calculating the peak area ratio of the response in the presence of matrix ions to the analyte peak response in the absence of matrix ions. Six different lots of blank rat plasma spiked with analyte at three levels were used for the evaluation. The recovery and matrix effect of the IS was measured in the same way.
2.5.5. Stability
The stability of poliumoside in rat plasma was investigated by analyzing triplicate QC samples at three concentrations level (low, middle and high), exposed to different conditions. The stability of the spiked samples were evaluated as the following aspects, 1) after three completed freeze-thaw cycles, 2) the exposure of the spiked samples at ice-water bath for 12 h, 3) room temperature for 12 h. For stability of poliumoside in processed samples, the prepared QC samples were placed in an auto-sampler at 4˚C for 3 days. This experiment can clearly determine the stability of the collected samples and the accurate analysis for determination.
2.6. Pharmacokinetic Study
Male Sprague-Dawley (SD) rats weighing 220 - 250 g were obtained from Laboratory Animal center of Jinan University (Guangdong, China). Rats were housed in a humidity-controlled room maintained on 12 h light-dark cycles for 5 days before the experiment. Standard animal food and water were provided ad libitum. Twelve rats were anaesthetized using 10% chloral hydrate and the jugular vein were cannulated for blood collection before 24 h of the experiment. Diet was prohibited for 12 h before the experiment, while water was freely taken. All procedures were approved by the Animal Care and Ethics Committee of Jinan University (Guangzhou, China).
Rats were randomly assigned to two groups (six rats per group). Poliumoside was dissolved in saline and administrated to rats by oral and intravenous injection. Approximately 0.3 mL blood samples were collected from jugular vein into 1.5 mL heparinized centrifuge tubes. Time points were set at 2, 5, 10, 20, 30, 45, 60, 90, 120, 180, 240, 360 min for the intravenous group (10 mg/kg) and 5, 10, 20, 30, 45, 60, 90, 120, 180, 240, 360, 480, 720 min for the oral group (200 mg/kg). Blood samples were immediately centrifuged (5000 g, 4˚C, 8 min), and 100 μL aliquot of supernatant (plasma) was collected and frozen at −80˚C before analysis.
3. Results and Discussion
3.1. Method Development and Optimization
MS parameters were optimized by directly infusing a standard solution into the ESI source. Both positive and negative ion mode were employed to confirm the accurate ions of poliumoside and IS. All analytes showed a better response under the negative mode. Ultimately, the m/z 769.2537 for poliumoside and 179.0328 for IS were used for the further analysis under negative ion mode. Other parameters have been showed in section 2.2.
To separate the interfering compounds from the poliumoside and IS, different columns and mobile phase compositions were compared for chromatographic separation. The Waters BEH C18 column showed better peak shape and appropriate retention time for the analyte than Phenomenex Kinetex C18 column (2.1 × 50 mm, 2.6 μm). Appropriate ionization and chromatographic behavior were based on the proper mobile phase [14] - [16] . The gradient mobile phase with water (containing 0.1% formic acid) and acetonitrile greatly shortened the retention time of the analyte. The gradient was verified to be stable and repeatable according to the following test of method validations. The addition of 0.1% formic acid in water and acetonitrile could improve peak shapes under the optimized conditions. The retention time of IS and poliumoside were 1.91 min and 1.73 min for pharmacokinetic study, respectively.
Appropriate extraction method of the plasma is essential for the quantitative analysis. The technique of liquid-liquid extraction and precipitation of protein was tested for alternative. The method of liquid-liquid extraction showed low recovery (30%) when ethyl acetate was used as extraction solvents, and this method was both complicated and time consuming. Various protein-precipitating reagents, such as methanol, acetonitrile and 10% TCA, were tested. Strong response value, good recovery and clear supernatant for the analytes were observed by using 10% TCA as the protein-precipitating reagent. Comparing with the neutral or alkaline conditions, poliumoside was found more stable in acidic solvents [11] . Analyte was more stable in 10% TCA (as acidic solvent) than in the other two precipitant at room temperature, so 10% TCA was elected to be the most suitable protein- precipitating reagent in the experiment.
3.2. Method Validations
3.2.1. Selectivity
Figure 2 presents typical chromatograms of blank plasma, the blank plasma sample spiked with poliumoside and IS, and test plasma sample obtained at 45 min after an intravenous administration of poliumoside. No significant interfering endogenous peaks were observed at the retention time of analyte and IS under the current chromatographic and MS conditions, demonstrating that this method can accurately differentiate and quantify the analyte of the plasma samples.
3.2.2. Linearity and LLOQ
The calibration standards of poliumoside (50, 100, 625, 1250, 2500, 5000, 10,000 ng/mL) were obtained by spiking blank plasma with appropriate amounts of working solutions to the final concentration range of 50 - 10,000 ng/mL. Figure 3 showed the calibration curve that was constructed by plotting the peak area ratio of poliumoside to IS versus concentrations of analyte. Linear equation of the calibration curve was: y = 0.0749x − 0.0054 (r2 = 0.993) within the concentration from 50 to 10,000 ng/mL. The LLOQ for the analyte was confirmed at 50 ng/mL and the limit of detection was found to be 13 ng/mL.
3.2.3. Precision and Accuracy
Precision and accuracy at three concentration levels of poliumoside were shown in Table 1. The intra-day and inter-day precision of QC samples were all less than 7.97%. The accuracy ranged from −7.01% to 3.36% for all the three concentration levels. The results were found to be within the acceptance criteria, demonstrating that the method were reproducible and reliable.
![]() (a) (b) (c)
(a) (b) (c)
Figure 2. Representative UPLC-MS chromatograms: (a) Blank plasma; (b) Blank plasma spiked with the poliumoside and IS; (c) A rat plasma sample of 45 min after intravenous administration of poliumoside (10 mg/kg).
![]()
Table 1. Precision and accuracy for poliumoside in rat plasma (n = 6).
![]()
Figure 3. The calibration curve of poliumoside at the concentration range of 50 - 10,000 ng/mL.
3.2.4. Recovery and Matrix Effect
The recovery and matrix effect of poliumoside and IS were shown in Table 2. The mean recovery of three concentrations was between 93.89% and 103.32%. Matrix effect is the suppression or enhancement of ionization of analytes by the presence of matrix components in the biological samples [17] . The matrix effect ranged from 92.49% to 105.34%. Partial data of recovery and matrix effects was greater than 100%, and this phenomenon may result from the ion enhancement effect of the matrix for poliumoside. We can conclude that no significant signal suppression or enhancement were observed in the present study. These results were within the acceptable range and indicated that the method were dependable.
3.2.5. Stability
Table 3 summarized all established stability for the analyte subjected to different conditions. The RE values were between −5.0% and 13.6% under low temperature conditions. The RE values of the analyte at room temperature were between −21.07% and −44.04%, indicating that poliumoside was unstable under this condition. The results demonstrated that the poliumoside samples kept stable under the low temperature conditions and it was also the guidance for the sample preparation disposing on the ice. Consequently, the developed method was suitable for the pharmacokinetic study of poliumoside.
3.3. Pharmacokinetic Study
The plasma concentration-time profiles of poliumoside were shown in Figure 4. The concentration of poliumoside was calculated using the following equation:
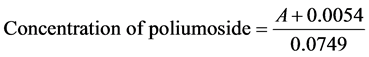
A is the peak area ratio of poliumoside to IS from different samples. The mean pharmacokinetic parameters from non-compartment modeling were listed in Table 4. All the pharmacokinetic parameters were calculated by PK solver software. The validated method was successfully applied to measure the plasma concentration of poliumoside in rats. After oral (200 mg/kg) administration of poliumoside, the Tmax was 30 min, suggesting that poliumoside was rapidly absorbed through the gastrointestinal (GI) tract. However, the calculated Cmax was only 561 ng/mL and the low absorbing may result from the high polarity of the structure of poliumoside. Plasma concentration experienced rapidly decline after peak concentration. The fast tissue distribution and the sluggish overall elimination may give rise to the result. The AUC0−t was 146 ± 9.88 μg/mL*min ,the mean residence time (MRT) of the analyte after oral and intravenous administration were 15.41 ± 4.17 h and 0.74 ± 0.29 h, respectively.
![]() (a) (b)
(a) (b)
Figure 4. (a) Mean plasma concentration time profile after intravenous (10 mg/kg) administration of poliumoside in rats; (b) Mean plasma concentration time profile after oral (200 mg/kg) administration of poliumoside.
![]()
Table 2. Recovery, matrix effect for poliumoside and IS of QC samples in rat plasma (n = 6).
![]()
Table 3. Stability of poliumoside under different conditions in rat plasma (n = 3).
![]()
Table 4. The mean pharmacokinetic parameters from non-compartment.
Abbreviations: AUC0−t, area under the concentration-time curve calculated from zero up to the last measured concentration; AUC0−∞, area under the concentration-time curve extrapolated from zero up to infinity; MRT, mean residence time of the analysis; Tmax, time to maximum observed concentartion; Cmax, maximum plasma concentration; t1/2, elimination; AUMC0−∞, area under the first moment of the concentration-time curve from zero up to infinity; V, volume of distribution; CL, clearance.
The systematic pharmacokinetics of poliumoside in rats was explored for the first time. Furtherly, the bioavailability of poliumoside was firstly reported to be 0.69%, which is similar to the other PhGs [18] - [20] . The metabolism of PhG in GI tract was largely reported, such as poliumoside [21] and echinacoside [22] [23] . The complex microbial ecosystem in GI is generally considered as a separated organ with powerful metabolic capacity. The metabolism in GI may directly decrease absorption of poliumoside and result in the low bioavailability ultimately.
4. Conclusion
In conclusion, a rapid, sensitive and selective UPLC/Q-TOF-MS method was established and applied for the determination of poliumoside in rat plasma. Only 3 minutes were needed for the analysis time, and this method truncated the detecting time and improved the accuracy to a large extent. The pharmacokinetic parameters of poliumoside in rats were successfully characterized in this study and the bioavailability was firstly reported to be 0.69%. This study provides a novel approach for pharmacokinetics of poliumoside in rat plasma and provides a basis for further pharmacology research.
NOTES
![]()
*Corresponding author.