Synthesis and Photophysical Properties of Fluorene or Carbazole-Based Alternating Copolymers Containing Si and Ethynylene Units in the Main Chain ()
Received 30 January 2016; accepted 12 March 2016; published 15 March 2016

1. Introduction
A number of conjugated polymers have been developed due to their attractive features, especially phot- and electro-luminescence. One of the most useful applications of the conjugated polymers must be emission layers for organic light-emitting diodes (LEDs) [1] [2] . There are some advantages in the polymer electro-lumines- cence in comparison with inorganic electro-luminescence. The first is process ability to form the emission layer. The conjugated polymers with specific molecular structures show good solubility in organic solvents, and spin- coating or casting of the solutions makes it possible to form thin emission layer in the LED devices. Molecular design of the conjugated polymers can improve the solubility of the polymers in conventional organic solvents, for examples, incorporation of bulky side group and/or copolymerization. The second is control of the emission wavelength by the molecular design of the polymers. The emission wavelength is correlated with the conjugation length in the polymer electro-luminescence. The shortening or extension of the conjugation length of the polymers tends to induce blue-shift or red-shift of the emission wavelength, respectively. The third is geometrical variation of the polymers, such as conjugated polymers with networked, dendritic, or branched structure.
Incorporation of Si atom in the main chain of the π-conjugated polymers is effective to shorten the conjugation length [3] - [14] . Kim et al. reported the incorporation of Si atom into poly(p-phenylenevinlene), which showed blue-emission [3] . Masuda et al. synthesized poly(phenylene-ethnylene) containing Si atom in the main chain [4] . They observed the emissions of the polymers derived from not only π-π* transition of the π-conjuga- tion but intramolecular charge transfer through σ-π conjugation. We developed fluorene- and carbazole-based alternating copolymers containing Si-vinylene units in the main chain [15] [16] . These copolymers were synthesized by alternating copolymerization of dibromofluorene or dibromocarbazole with Si containing divinyl or diallyl compounds using Mizoroki-Heck reaction with a Pd catalyst. Incorporation of Si and unsaturated vinylene units in the main chain of the conjugated polymers was effective to improve solubility in the organic solvents. The incorporation of Si or vinylene units in the π-conjugated polymers played the opposite role for their photophysical properties. That is, the Si units shorten and vinylene units extend the conjugation length. The fluorene- and carbazole-based alternating copolymers containing Si-vinylene units in the main chain showed both the blue-shift and red-shift in the absorption and luminescence peaks depend on the state of the copolymers. As the next step, we came to an idea to incorporate Si-ethynlene units in the corresponding copolymers.
The conjugated network polymers with the σ-π conjugation have been synthesized by some synthetic methods [6] [17] - [19] . Neckers et al. synthesized dendritic poly(p-phenylenevinlene) containing Si-vinylene units, and reported the relationship between length of the p-phenylenevinlene unit and quantum yield [17] . Yamashita et al. synthesized σ-π conjugated network polymers by a hydrosilylation reaction between dihydrosilane compounds and diethynyl/triethynyl-benzene mixture, and reported increase of the absorption and fluorescence intensities with increasing of the crosslinking density [6] . Ishikawa et al. reported synthesis of the σ-π conjugated network copolymers by a crosslinking of poly[(silylene)diethnylene] with 1,4-bis(methylphenylsilyl)benzene using a hydrosilylation reaction [19] . This method is usable to synthesize the network polymers with the σ-π conjugation from the fluorene- and carbazole-based copolymers having Si-ethynlene units in the main chain synthesized in this report.
This paper reports the alternating copolymerization of 9,9-dihexyl-2,7-dibromofluorene (HFl) or N-hexyl-2,7- dibromocarbazole (HCz) with diethynylene Si compounds, diethynyldimethylsilane (EMS) or diethynyldiphenylsilane (EPS), by Sonogashira coupling reaction, as shown in Scheme 1, and the photophysical properties of the resulting copolymers. We also synthesize the network polymers by a hydrosilylation reaction of the obtained copolymers with 1,4-bis(dimethylsilyl)benzene (DMSB), and study the effect of the networking on the photophysical properties in comparison with the original linear copolymers.
2. Experimental
2.1. Materials
Dichlorodimethylsilane (Aldrich Co. Ltd.), dichlorodiphenylsilane (Tokyo Chemical Industry Co. Ltd.), and tetrahydrofuran (THF) solution of ethynylmagnesiumbromide (0.5 M) (Aldrich Co. Ltd.) were purchased and used as received. Palladium(II)dichlororbis(triphenylphosphine) (PdCl2(PPh3)2) (Wako Pure Chemical Industries, Ltd.), CuI (Wako Pure Chemical Industries, Ltd.), triphenylphosphine (PPh3) (Wako Pure Chemical Industries,
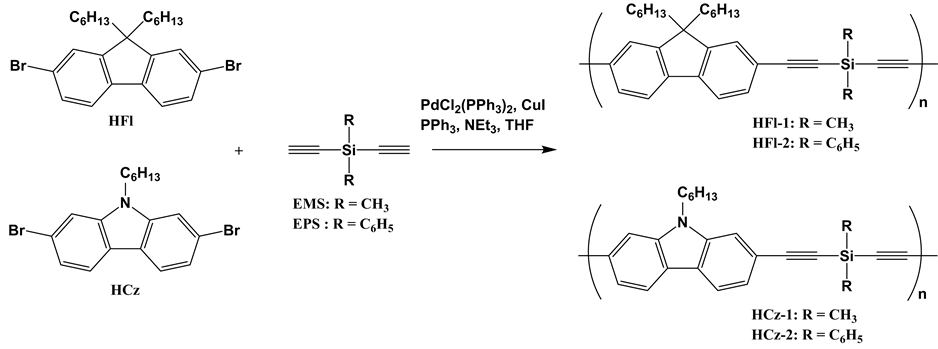
Scheme 1. Synthesis of fluorere or carbazole-based lwternating copolymers containing Si and ethynylene units in the main chain.
Ltd.), THF (dehydrate grade, Kanto Chemical Co. Ltd.), and diethylether (dehydrate grade, Kanto Chemical Co. Ltd.) were commercially obtained, and used without further purification. Triethylamine (NEt3) (Kanto Chemical Co. Ltd.) was dried with CaH2 and distilled under a nitrogen atmosphere. HFl (Aldrich Co. Ltd.) was commercially obtained and used as received. HCz was synthesized according to the literatures [20] . Chloroform for photophysical properties analyses (Kanto Chemical Co. Ltd.) and tris(8-hydroxyquinoline)aluminum (Alq3) (Wako Pure Chemical Industries, Ltd.) were commercially obtained, and used without further purification. DMSB was commercially obtained from Chisso Co. Ltd., and used as received. Toluene (Kanto Chemical Co. Ltd.) was dried over calcium hydride under refluxing for 6 h, and distilled under nitrogen atmosphere before use. Platinum-divinyltetramethyldisiloxane complex (Pt(dvs)) in vinyl terminated polydimethyl siloxane (Chisso Co. Ltd.) was diluted to 0.60 mM with distilled toluene and stored under nitrogen atmosphere.
2.2. Synthesis of EMS and EPS
A THF solution of ethynylmagnesiumbromide (0.5 M, 80 mL) was added dropwise to a diethylether solution (20 mL) of dichlorodimethylsilane, or dichlorodiphenylsilane (0.02 mol) at room temperature. The reaction mixture was refluxed at 40˚C for 2 h, and cooled to room temperature. After the precipitates were filtered off, the solution was concentrated by evaporation. The obtained product was extracted with n-pentane, and n-pen- tane was removed off by evaporation under reduced pressure. The obtained products were pure enough by 1H NMR spectroscopy. Yields of EMS and EPS were 52% or 65%, respectively.
2.3. Copolymerization
The copolymerization was carried out in a 100 mL glass reactor equipped with a magnetic stirrer. HFl or HCz (0.718 mmol), CuI (6.53 μmol), PPh3 (6.53 μmol) were added to the reactor under nitrogen atmosphere. EMS or EPS (0.718 mmol), THF solution (2.5 mL) of PdCl2(PPh3)2 (25.3 μmol) and NEt3 (0.04 mmol) were introduced to the reactor, and the copolymerization was conducted at 60˚C for 48 h. The copolymerization was terminated by adding a small amount of methanol. The polymer was precipitated in a large excess of methanol and recovered by filtration or decantation. The copolymer obtained was dissolved in chloroform and re-precipitated in methanol. The precipitate was recovered by filtration or decantation and dried in vacuo at 60˚C for 6 h.
2.4. Synthesis of Network Copolymers
The reaction was carried out in a 100 mL glass reactor equipped with a magnetic stirrer. A copolymer (0.410 mmol of repeating unit), DMSB (0.205 mmol), and toluene solution of Pt(dvs) (10.4 μmol) were added to the reactor under a nitrogen atmosphere. The copolymerization was terminated by adding a small amount of methanol. The polymer was precipitated in a large excess of methanol and recovered by filtration. The precipitate was recovered by filtration and dried in vacuo at 60˚C for 6 h.
2.5. Analytical Procedures
1H and 13C NMR spectra of the copolymers were recorded at room temperature on a JEOL-JNM-LA300 spectrometer in pulse Fourier transform mode. The sample solution was made in CDCl3 as a solvent, and the resonance of CDCl3 (7.24 ppm) was used as an internal reference. Molecular weight and molecular weight distribution of the copolymers were measured at 40˚C by means of gel-permeation chromatography, SHIMADZU Prominence GPC System, using chloroform as a solvent and calibrated with standard polystyrene samples. UV-vis absorption spectroscopy was conducted with SHIMADZU UV-1600PC in a chloroform solution, 10−5 mol/L of the repeating unit of the copolymers. Photoluminescence (PL) spectroscopy was investigated with a SHIMADZU RF-1500 in a chloroform solution, 10−8 mol/L of the repeating units of the copolymer, or cast film from a chloroform solution, excited at the maximum absorption wavelength of the copolymers.
3. Results and Discussion
3.1. Synthesis of Alternating Copolymers
Copolymerization of HFl, HCz and EMS, EPS has been investigated with PdCl2(PPh3)2 in THF at 60˚C (Scheme 1). The results are summarized in Table 1. Although the molecular weights of the copolymers were relatively low, the corresponding copolymers were obtained in good yield. All the copolymers were soluble in conventional solvents such as acetone, chloroform, THF, toluene, and dimethylformamide.
3.2. Photophysical Properties of HFl-Based Copolymers
Photophysical properties of the HFl-based copolymers in chloroform solution were investigated with UV-vis absorption or PL spectroscopy. The UV-vis spectra of the HFlu-based copolymers in chloroform are shown in Figure 1. The copolymers showed a strong absorption peaks at 280 nm with broad shoulder peaks at around 370 nm. The absorption peaks at around 370 nm should be derived from π-π* transition of the fluorene moiety, as detected in the reference polymer HFl-0. The absorption peaks at 280 nm, which was not detected in the reference polymer HFl-0, would be induced by intramolecular charge transfer through the σ-π moiety in the copolymers, as previously reported [15] . All the absorption peaks of the present copolymers containing Si-ethynylene units were blue-shifted in comparison with those of the corresponding copolymers containing Si-vinylene units (HFl-i, HFl-ii) or HFlu homo polymer (HFl-0) (Scheme 2, Table 2). The blue-shift in UV-vis spectra of the
![]()
Table 1. Results of copolymerization of HFl,r HCz and EMS, EPSa.
aHFl, HCz = 0.718 mmol, EMS, EPS = 0.718 mmol, THF = 2.5 mL, PdCl2 (PPh3)2 = 25.3 μmol, PPh3 = 6.53 μmol, NEt3 = 0.04 mmol, CuI = 6.53 μmol, 60˚C, 48 h, bDetermined by gel permeation chromatography using polystyrene standards.
![]()
Table 2. Photophysical properties of HFl-based copolymers containing Si-unsaturated units in the main chaina.
aEvaluated in chloroform (repeating unit: 10−5 mol/L), bracket: shoulder peak, bEvaluated in chloroform (repeating unit:10−8 mol/L), cEmission was excited at λmax of absorption, dQuantum efficiency measured using Alq3 as a standard, ePolymer was casted from a chloroform solution (repeating unit: 10−3 mol/L), fRef [15] .
![]()
![]()
Scheme 2. HFl- and HCz-based (co)polymers for reference.
![]()
Figure 1. UV spectra of (a) HFl-1, (b) HFl-2, and (c) HFl-0 (reference) in chloroform solution, HFl unit: 10−5 mol/L.
present copolymers should be derived from shortening of the conjugation length by Si-ethynylene unit having short length and rigid structure.
Figure 2 shows the PL spectra of the HFl-based copolymers in chloroform. The copolymers showed broad emission peaks at around 415 nm. The emission wavelengths of the copolymers were almost same with the HFl homo-polymer (HFl-0). One explanation of the results is that both the shortening with Si and extension with ethynylene units of the π-conjugation length by σ-π moiety would cancel the shifts of the emission wavelength. The excitation spectra of the HFl-1, which were detected at the emission wavelength of 415 nm, showed the maximum emission intensity with excitation wavelength of 279 nm, which was λmax of the absorption spectrum. The excitation with 372 nm, which was the shoulder peak of the absorption spectrum, just induced a weak emission at 415 nm. These results indicate that the emission at 415 nm should be derived from the intramolecular charge transfer through the σ-π moiety. The PL quantum yields of HFl-based copolymers, 0.9 (HFl-1) and 0.2 (HFl-2), were in range those of the reference (co)polymers. The PL spectroscopy of the copolymers was also investigated in the solid state, as the cast films prepared form the chloroform solutions at room temperature. The PL spectra of the HFl-1 and HFl-2 in the solid state are shown in Figure 3. The copolymers showed broad emission peaks at 380 nm, which was blue-shifted in comparison with that of HFl-0. Furthermore, the emission wavelengths in the solid state were blue-shifted in comparison with those in the chloroform solutions. These results
![]()
Figure 2. PL spectra of (a) HFl-1, (b) HFl-2, and (c) HFl-0 (reference) in chloroform solution, HFl unit: 10−8 mol/L.
![]()
Figure 3. PL spectra of (a) HFl-1, (b) HFl-2, and (c) HFl-0 (reference) in solid state (cast film).
indicate that the aggregation of the HFl-1 and HFl-2 copolymers would form new high energy states. The reference copolymers with Si-vinylene units (HFl-i and HFl-ii) and homo-polymer HFl-0 (Scheme 2) showed the opposite tendency [15] . The Si-ethynylene units in the present copolymers should play an important role for the blue-shift of the emission wavelength in the solid state.
3.3. Photophysical Properties of HCz-Based Copolymers
Photophysical properties of the HCz-based copolymers in chloroform solution were studied with UV-vis absorption or PL spectroscopy. Figure 4 shows the UV-vis spectra of the HCz-based copolymers in chloroform. The chloroform solutions of the copolymers showed strong absorption peaks at 270 nm with broad shoulder peaks at around 360 - 380 nm. The absorption peaks at around 360 - 380 nm should be derived from the π-π* transition of the carbazole moieties, as detected in the reference polymer HCz-0 [15] . The absorption peaks at 270 nm would be induced by the intramolecular charge transfer through the σ-π moiety, as shown in the HFl- based copolymers described above. The peak intensities of the shoulder peaks of the HCz-based copolymers were higher than those of the HFl-based copolymers. The result indicates that the intramolecular charge transfer through the σ-π moiety should occur frequently in the HCz-based copolymers. All the absorption peaks of the present HCz-based copolymers containing Si-ethynylene units were blue-shifted in comparison with those of the corresponding copolymers containing Si-vinylene units (HCz-i, HCz-ii) or HCz homo-polymer (HCz-0) (Scheme 2, Table 3). The blue-shift in UV-vis spectra of the present copolymers should be derived from shortening of the conjugation length by Si-ethynylene units, as observed in the HFl-based copolymers.
Figure 5 shows the PL spectra of the HCz-based copolymers (HCz-1 and HCz-2) in chloroform. The copolymers showed broad emission peaks at around 425 nm. The emission wavelengths of the copolymers were red-shifted (about 10 nm) in comparison with that of the HCz homo-polymer (HCz-0). The extension of π-conjugation length with ethynylene unit would be more effective than the shortening of it with Si by the σ-π moiety in the HCz-based copolymers. The PL quantum yield of HCz-based copolymers, 0.2 (HCz-1) and 0.5 (HCz-2), were lower than the reference (co)polymers. Figure 6 shows the PL spectra of the HCz-based copolymers in the solid state. The copolymers showed broad emission peaks at 360 nm, which were blue-shifted in comparison with that of HCz-0. The emission wavelengths in the solid state were blue-shifted in comparison with those in the chloroform solutions, as observed in the HFl-based copolymers. The reference copolymers with Si-vinylene units (HCz-i and HCz-ii) and homo-polymer HCz-0 (Scheme 2) showed the opposite tendency [15] .
![]()
Table 3. Photophysical properties of HCz-based copolymers containing Si-unsaturated units in the main chaina.
aEvaluated in chloroform (repeating unit: 10−5 mol/L), bracket: shoulder peak, bEvaluated in chloroform (repeating unit:10−8 mol/L), cEmission was excited at λmax of absorption, dQuantum efficiency measured using Alq3 as a standard, ePolymer was casted from a chloroform solution (repeating unit: 10−3 mol/L), fRef [15] .
![]()
Figure 4. UV spectra of (a) HCz-1, (b) HCz-2, and (c) HCz-0 (reference) in chloroform solution, HCz unit: 10−5 mol/L.
![]()
Figure 5. PL spectra of (a) HCz-1, (b) HCz-2, and (c) HCz-0 (reference) in chloroform solution, HCz unit: 10−8 mol/L.
![]()
Figure 6. PL spectra of (a) HCz-1, (b) HCz-2, and (c) HCz-0 (reference) in solid state (cast film).
The results should be derived from the specific aggregation of the copolymers induced by Si-ethynylene units in the copolymers, as explained in the results of the HFl-based copolymers. The blue-shift factors, difference of the emission wavelengths in the chloroform solution and solid state, of the HCz-based copolymers (about 70 nm) were higher than those of the HFl-based copolymers (about 30 nm). The results indicate that the molecular structure of the HCz unit should be also important to form the new high energy state in the solid state. We have tried to investigate the solid structure of the copolymers by X-ray diffraction, although, we couldn’t have the clear diffraction patterns of the copolymers. It remains as unsettled question what makes difference of the blue-shift factor between the HFl and HCz units in the copolymers. One possibility is that HCz units in the copolymers would effectively prevent from formation of the π-stacking, which causes red-shift of the emission wavelength, due to the electric repulsion between nitrogen atoms in the carbazole moiety.
3.4. Synthesis and Photophysical Properties of Network Copolymers with DMSB
The HFl and HCz based network copolymers were prepared by a hydrosilylation reaction with DMSB using Pt(dvs) catalyst, as shown in Scheme 3. The resulting network copolymers were soluble in chloroform, and insoluble part was hardly detected. The result indicates that the reaction of the copolymers with low molecular weights could not form the infinite network structure and yielded the branching copolymers. The degree of the
![]()
Scheme 3. Synthesis of HFl, HCz-based network cpolymers using a hydrosilylation reaction with DMSB.
![]()
Table 4. Photophysical properties of HCz-based copolymers containing Si-unsaturated units in the main chaina.
aHFl, HCz: repanting units = 0.41 mmol, DMSB = 0.205 mmol, Pt(dvs) = 10.4 μmol (0.60 mM of toluene solution), 60C, 6 h, bDetermined by 13C NMR spectrum, bracket: shoulder peak, cEvaluated in chloroform (10−5 mol/L), bracket: shoulder peak, dEvaluated in chloroform (repeating unit: 10−8 mol/L), bracket: shoulder peak, emission was excited at λmax of absorption.
*Corresponding author.
The fluorene or carbazole-based alternating copolymers containing Si and ethynylene units in the main chain were successfully synthesized by Sonogashira coupling reaction of dibromo-fluornene or carbazole (HFl, HCz) and diethynylene Si compounds (EMS, EPS). The copolymers showed good solubility in conventional organic solvents such as acetone, chloroform, THF, toluene, and dimethyl foramide. The resulting copolymers showed bimodal absorption peaks and broad emission peaks in the chloroform solutions derived from the intramolecular charge transfer through the σ-π moiety. The emission peaks of the copolymers in the solid state showed remarkable blue-shift in comparison with those of the corresponding homo-polymers. The blue-shift in the emission peaks of the solid state copolymers should be induced by the higher energy states derived from intramolecular charge transfer through the σ-π moiety in the copolymer. The phenomenon was not observed in the reference (co)polymers. The Si-ethynlene unit in the copolymers should play an important role for the specific molecular aggregation of the copolymers in the solid state. The hydrosilylation reaction of the ethynyl groups in the copolymers with DMSB yielded the branched copolymers, which were soluble in organic solvents. The reaction of HCz-based copolymers with DMSB induced the emission at short wavelengths in the chloroform solution.
The present copolymers should be one of the useful conjugated polymers with fluorescence features. The modifications and reactions of the unsaturated ethynyl groups in the copolymers would enable us to control the wavelength in the emission and to form the conjugated polymers with geometrical variations. As the next step, we are looking carefully into the molecular structure of the copolymers in the solid state. We are also trying to apply the copolymers as emission layers for LEDs, and the results will be reported elsewhere.
NOTES
![]()
*Corresponding author.