Comparative Study of Removal of Hexavalent Chromium from Water Using Metal Oxide Nanoparticles ()
Received 28 December 2015; accepted 13 February 2016; published 16 February 2016

1. Introduction
Chromium is widely used by many industries and released into environment through various industrial processes including metal finishing industry, iron and steel industries and inorganic chemicals production, tanneries etc. High concentration of chromium in water may cause hazard to the environment [1] . Hexavalent chromium and its compounds are highly toxic and are considered as a carcinogen and mutagens [2] . The other effects of chromium(VI) on human health include lung cancer, liver, kidney and gastric damage, and epidermal irritation and sensitization [3] . Most surface water contains 1 to 10 µg/liter of hexavalent chromium. The current guideline as per WHO value is 0.05 mg/liter (WHO/SDE/WSH/03.04/04).
Various treatments employed for the removal of chromium from water include reduction, precipitation, ion- exchange and solvent extraction etc. However, these methods are ineffective and require high energy for operation. Adsorption is considered as the most effective and widely used technique due to high removal efficiency, simplicity and low cost [4] .
Many adsorbents have been reported for the removal of hexavalent chromium from water such as activated carbon (AC) [5] [6] , activated alumina [7] , chitosan polymer [8] , zeolite [9] , low cost bio-adsorbent such as olive, leaves, wool etc. [10] , saw dust [11] , rice husk, wheat bran [12] , bentonite [13] , metal oxides such as ferric hydroxide, Fe [14] ion exchange resin [15] , nanostructured adsorbents [16] etc. Most of the adsorbents have low adsorption capacity and poor stability. The nature of adsorbent is mainly responsible for the chromium removal from water.
Nanoporous and nanostructured materials have unique surface area, structural and bulk properties. Because of these properties nanomaterials have important applications in environmental remediation and water purification. The most commonly used nanoparticles are nanoscale zero-valent iron which has also been reported for treatment of several wastewater contaminants including nutrients, organic pollutants, metals etc. The major limitation with existing nanopartcles is agglomeration and non-selectivity [17] . In this work different classes of adsorbents were synthesized and evaluated for chromium removal from water. Objective of the study was to synthesize copper oxide nanoparticles (CuNP) and aluminum oxide nanoparticles (AlNP) and compare them for removal of hexavalent chromium from water.
2. Material and Method
2.1. Synthesis of Copper Oxide Nanoparticles (CuNP)
All the chemicals used in this study were of analytical grade and procured from E-Merck India Ltd and Aldrich. The important chemicals used were copper nitrate, aluminium nitrate and monohydrate citric acid. Deionised water of highest purity was used throughout the study. CuNP was synthesised by taking 0.1 M of Copper Nitrate used as a precursor solution and mono hydrated citric acid (Merck 99.5%) as a gelating agent in appropriate amount of deionised water. The solution was then heated on hot plate and temperature was maintained to 85˚C to 90˚C. The dried gel was calcined at temperatures of 500˚C for 4 h. The calcined material was again washed with DI water and oven dried for 3 - 4 h. CuNP were prepared using different concentrations of copper nitrate and it was observed that 0.1 M concentration of copper gave maximum removal efficiency and were selected for further studies.
2.2. Aluminum Oxide Nanoparticles (AlNP)
The same synthesis protocol was repeated as mentioned for the synthesis of CuNP except for using Aluminum Nitrate salt as precursor instead of Copper Nitrate.
2.3. Characterisation of CuNP and AlNP
The CuNP and AlNP were thoroughly characterized to study the structure, morphology and composition of CuNP and AlNP by using techniques such as XRD, FTIR and TEM etc. The X-ray diffractometer (Model Rigaku: Miniflex) was used for identification of phases and crystalline species of CuNP and AlNP. The powdered sample was scan for 2θ ranges from 10˚ to 90˚. Transmission electron microscopy (TEM) analysis was carried out by JEOL instrument TEM Microscope (JSM 100 CX). Fourier transform infrared spectrometer (Bruker, Model Vertex) was used to determine the interaction of Cr with adsorbent.
2.4. Adsorption Studies
Initial solution of 5 mg/L of Cr(VI) was prepared from 1000 mg/L of chromium(VI) stock solution. In batch adsorption process 1 to 10 g/L dose of adsorbent were taken in 250 ml polycarbonate conical flasks. The flasks were kept in the orbital shaking incubator for 24 hours @ 150 rpm at 27˚C ± 1˚C. The pH of sample was maintained 6.5. After 24 hours shaking time the flask were withdrawn from the shaker and the adsorbent was separated by centrifugation and the supernatant was used for analysis of residual Cr(VI) concentrations using ICP- MS (Perkin Elmer, Nexion 300). The NIST standards supplied by Sigma-Aldrich were used for calibration. The experiments were repeated twice and it was observed that the experimental error was within ±2%.
The amount of Cr(VI) adsorbed (mg∙g−1) at time t was computed using following equation [18] :
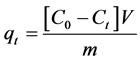 (1)
(1)
where, C0 is the initial concentration of Cr(VI), Ct is the concentration of Cr(VI) after adsorption at time t, V is the volume of solution used and m is the mass of adsorbent.
3. Discussion
3.1. Characterization of Adsorbent
The PXRD patterns of CuNP and AlNP are presented in Figure 1(a) and Figure 1(b). The sharp peaks obtained in Figure 1(a) at 2θ = 35.46˚ and 38.66˚ shows crystalline nature of the CuNP and confirm the formation of CuO (PDF 89-2529) phases. The PXRD pattern of AlNP shown in Figure 1(b) revealed the amorphous nature of AlNP and no sharp peak was obtained. The broad peak observed between 20˚ to 30˚ may be assigned to Al2O3 phase.
Transmission Electron Microscopy (TEM) was carried out to determine the size and morphology of synthesized nanoparticles. The diameter of particles shows that most of the particles are below 100 nm, however the accurate shape and size of particles could not be determined as the particles are agglomerated. High resolution image of AlNP (Figure 2(a)) shows nano-crystalline core coated with amorphous Al2O3. In 20 nm magnification the nanoparticles (Figure 2(b)) are clearly visible. The average diameter of the particles is about 20 to 50 nm and most of the particles are seen as aggregates. The SAED ring pattern in Figure 2(c) is indicated the amorphous nature of AlNP.
The TEM of CuNP is presented in Figure 3(a) and Figure 3(b). High resolution image of CuNP Figure 3(a) shows the particles aggregate on porous surface. The size and shape of particle depicted in Figure 3(b) confirms the particle size of about 30 to 60 nm and are nodular in shape. The SEAD pattern of CuNP shown in Figure 3(c) is dotted structure which confirms the crystalline nature of material.
The FTIR spectra of CuNP and AlNP were obtained in the range of 400 - 4000 cm−1 with a resolution of 1 cm−1 presented in Figure 4(a) and Figure 4(b). In order to identify possible interaction and functional groups present on the surface of CuNP and AlNP FTIR studies were performed. FTIR studies were performed before and after Cr(VI) adsorption on CuNP and AlNP. The FTIR spectrum of CuNP show some major peaks at 1019, 1593, 1735 cm−1. The band corresponds to the 1513, 1539 and 1735 observed in CuNP and AlNP which normally of metal bonding. The peaks obtained at 3716 to 3798 in CuNP and AlNP is attributed due to OH stretching vibration.
![]()
![]() (a) (b)
(a) (b)
Figure 1. (a) XRD of CuNP; (b) XRD of AlNP.
![]()
![]() (a) (b)
(a) (b)
Figure 4. (a) FTIR of CuNP; (b) FTIR of AlNP.
3.2. Batch Adsorption Study
Effect of Adsorbent Dose
The batch adsorption study of CuNP and AlNP were carried out to see the maximum adsorption at optimum dose with respect to contact time. The initial concentration of Cr(VI) was used 5 mg/l and pH was maintained 6.5. It was observed that the removal efficiency of AlNP was 98.3% at adsorbent dose of 10 g/L as compared to CuNP which exhibit 60% removal at same dose. The effect of adsorbent dose on adsorption efficiency of both adsorbent is shown in Figure 5.
4. Results
Adsorption Isotherms
In order to study the adsorption behaviour and to calculate adsorption capacity, Langmuir and Freundlich adsorption models were used. Langmuir isotherm model is most widely used isotherm applicable to monolayer adsorption on identical sites [19] . The Langmuir adsorption model can be represented in linear form as follows.
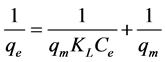 (2)
(2)
The adsorption capacity Qmax and energy of adsorption (k) were calculated from the slope and intercept of the Langmuir plot and found to be 1.93 mg/g for AlNP. Similarly the adsorption capacity obtained for CuNP was 19.61 mg/g.
The Freundlich model indicates heterogeneous adsorption on the adsorbent surface and considers as a multilayer adsorption. It is given by equation [20] .
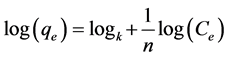 (3)
(3)
where qe is the adsorption capacity and n is the Freundlich constant related to adsorption intensity, Ce is the equilibrium concentration of adsorbate in solution (mg・l−1).
The experimental data and Langmuir and Freundlich adsorption isotherm fits for adsorption of Cr(VI) on AlNP and CuNP are presented in Figure 6(a) & Figure 6(b) and Figure 6(c) & Figure 6(d) respectively. The isotherm parameters are given in Table 1. On comparison of the fitness of the adsorption isotherm, Langmuir model is more appropriate for adsorption of Cr(VI) on AlNP.
5. Conclusion
The nanoparticles of CuNP and AlNP were synthesized and evaluated for removal of Cr(VI) from synthetic water. On comparison of the adsorption of Cr(VI) on copper oxide and aluminium oxide nanoparticles, it was
![]()
Figure 5. Adsorption of Cr(VI) on CuNP and AlNP.
![]()
Table 1. Adsorption isotherm parameters for chromium adsorption on AlNP and CuNP.
observed that AlNP exhibit excellent efficiency for the removal of Cr(VI). The PXRD analysis confirms the synthesis and presence of aluminium oxide and copper oxide phases. TEM images of CuNP and AlNP also confirm the formation of nanoparticles. The AlNP showed significantly high adsorption capacity for removal of Cr(VI) from water as compared to CuNP.
NOTES
![]()
*Corresponding author.