Adsorptive Treatment of Textile Wastewater Using Activated Carbon Produced from Mucuna pruriens Seed Shells ()
Received 6 October 2015; accepted 11 January 2016; published 18 January 2016

1. Introduction
Color is the first pollutant to be known in wastewater [1] . Water pollution due to discharge of coloured effluents from textile dye manufacturing and textile dyeing mills is one of the major environmental concerns in the world today [2] . The total dye consumption of the textile industry worldwide is more than 107 kg/year [3] . Congo red (CR) is benzedene based anionic dye [4] and malachite green (MG) is a cationic dye [5] . In aqueous solution, anionic dyes carry a net negative charge due to the presence of sulphonate (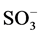 ) groups, while cationic dyes carry a net positive charge due to the presence of protonated amine or sulfur containing groups [6] . Many dyes may cause allergic dermatitis, skin irritation, dysfunction of kidney, liver, brain, reproductive and central nervous system [7] [8] . The aquatic ecosystem can also be affected due to the toxicity of these dyes. A very low concentration of dye can make water unacceptable for various purposes [9] . Various techniques have been employed for the removal of textile dyes from wastewaters which include adsorption, nano-filtrtion, electro kinetic coagulation, coagulation and precipitation, advanced chemical oxidation, electrochemical oxidation, ozonation, supported liquid membrane, liquid-liquid extraction and biological process [10] . Adsorption has been shown to be one of the most promising and extensively used methods for the removal of both inorganic and organic pollutants from contaminated water [11] . The use of activated carbon has been highlighted as an effective technique for dye removal due to its unique molecular structure, high porosity and an extensive surface area [12] . Mucuna pruriens (Figure 1) is a tropical legume known as velvet bean or cowitch and by other common names, found in Africa, India and the Caribbean. The plant is an annual, climbing shrub with long vines that can reach over 15 m in length. The endocarp of Mucuna pruriens is non toxic. Mucuna pruriens is called ukpo by the Ibos in the southeast of Nigeria [7] . It is usually used as food thickeners [7] .
) groups, while cationic dyes carry a net positive charge due to the presence of protonated amine or sulfur containing groups [6] . Many dyes may cause allergic dermatitis, skin irritation, dysfunction of kidney, liver, brain, reproductive and central nervous system [7] [8] . The aquatic ecosystem can also be affected due to the toxicity of these dyes. A very low concentration of dye can make water unacceptable for various purposes [9] . Various techniques have been employed for the removal of textile dyes from wastewaters which include adsorption, nano-filtrtion, electro kinetic coagulation, coagulation and precipitation, advanced chemical oxidation, electrochemical oxidation, ozonation, supported liquid membrane, liquid-liquid extraction and biological process [10] . Adsorption has been shown to be one of the most promising and extensively used methods for the removal of both inorganic and organic pollutants from contaminated water [11] . The use of activated carbon has been highlighted as an effective technique for dye removal due to its unique molecular structure, high porosity and an extensive surface area [12] . Mucuna pruriens (Figure 1) is a tropical legume known as velvet bean or cowitch and by other common names, found in Africa, India and the Caribbean. The plant is an annual, climbing shrub with long vines that can reach over 15 m in length. The endocarp of Mucuna pruriens is non toxic. Mucuna pruriens is called ukpo by the Ibos in the southeast of Nigeria [7] . It is usually used as food thickeners [7] .
Adsorption studies have been made using different agro based adsorbents on the removal of textile dyes such as Formosa papaya seed powder [13] , cow bone [14] , mangrove bark [3] , ginger waste [5] , and peanut shells [6] .
The objective of this study is to investigate the isotherm, kinetics, and thermodynamics on congo red and malachite green removals using an abundant waste in Southeast Nigeria. The removal efficiencies of the activated Mucuna pruriens seed shells on the dyes at the same conditions will be compared.
2. Experimental Materials and Methods
2.1. Preparation of the Adsorbent
Mucuna pruriens seed shells (MSS) were collected from Mucuna pruriens seed processing, Ogbete market, Enugu state, Nigeria and washed thoroughly with de-ionized water to remove dirt, dried in the oven at 105˚C. The MSS were ground, sieved to the desired particle size of 1 - 2 mm and soaked in 60% orthophosphoric acid (H3PO4) acid for 24 hours at room temperature and carbonized at 500˚C for 2 hours using muffle furnace (Model SX-2.5-10). The carbonized samples were washed with de-ionized water until pH 7, filtered and dried in the
![]()
![]() (a) (b)
(a) (b)
Figure 1. (a) (b) Mucuna pruriens seeds and shells respectively.
oven at 105˚C for 8 hours. The dried sample was allowed to cool to room temperature, sieved to different particle sizes and stored in air tight container for adsorption studies.
2.2. Characteristics of the Adsorbents
The physicochemical properties of the adsorbent (AMSS) were determined using the standard methods described by [15] . X-ray Fluorescence (XRF) was used to determine the oxides present in the Mucuna pruriens seed shells (MSS) using XRF spectrometer (Munipal 4 model). The Fourier transforms infrared (FTIR) was used to determine the functional groups present in MSS and AMSS using Shimadzu S8400 spectrophotometer, with samples prepared by the conventional KBr disc method. X-ray diffraction (XRD) was used to determine the diffraction pattern and inter planar spacing of AMSS. XRD patterns of the carbon was obtained on a powder X-ray diffractometer (Stchmabzu model 6000) with CuKα radiation having a scanning speed of 8.000 deg/min and tested at 40.0 kV and 30 mA. The patterns were recorded over a 2-theta (2θ) range of 2.0000 - 60.0000 deg. Scanning Electron Microscopy (SEM) was used to determine the surface morphology of MSS and AMSS.
2.3. Adsorption Studies
Congo red (CR) and malachite green (MG) of analytical grade were used without further purification. The reagents were of high grade. 0.1 g of each dye was dissolved in 1000 ml of de-ionized water to get a dye solution of 100 mg/l. Dye adsorption experiments were performed by taking 100 ml stock solution of dye and treated with a known dose of adsorbent at 120 rev/min. After a desired time of treatment, the dye solution was centrifuged and the concentration of the residue was determined using UV-Vis spectrophotometer (Model UV 754) at wavelength, λmax of 498 and 617 nm for CR and MG respectively. The effects of particle size, pH, adsorbent dose, concentration and contact time on the process of adsorption were investigated in the range of 0.3 - 1.5 mm, 2 - 10, 0.2 - 2.0 g, 100 - 500 mg/l and 10 - 150 min respectively. The percentage adsorbed was investigated by varying a parameter and keeping the other parameters constant. The amount of dye adsorbed per unit mass of activated carbon (mg/g) and percentage adsorbed (%) were obtained as follows:
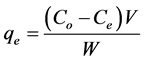 (1)
(1)
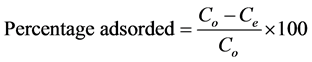 (2)
(2)
where Co is the initial concentration of dye in the solution (mg/l); Ce is the final concentration of dye in the solution (mg/l); V is the volume of the solution (l); and W is the weight of activated carbon (g).
The correlation coefficient (R2) was used to determine the conformity, applicability and acceptance of the isotherm and kinetic models. The sum of the squares error (SSE %) was also used to determine the adequacy of fit of the isotherm models. The higher the R2 value and the lower the value of SSE (%) are, the better the conformity and adequacy of fit of the model are. The SSE was estimated using the expression [16] :
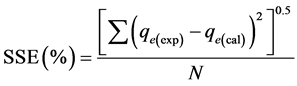 (3)
(3)
where N is the number of the data points used in each kinetic model plot.
3. Results and Discussion
3.1. Physicochemical Properties of the Adsorbents
The physicochemical properties of the adsorbent are presented in Table 1. The AMSS was found to have a high surface area indicating its high adsorptive performance. The adsorbent was also found to have a high percentage fixed carbon and iodine number. The pH value is near neutral which will be helpful for the treatment of all cases of dye waste water and the carbons can also be used for drinking water purification [17] . The activated carbon
![]()
Table 1. Physicochemical properties of AMSS.
was found to have low ash content (5.38%). If the ash content is high, it will interfere with the pore structure development and hence adsorption will be less [18] . X-ray Fluorescence (XRF) was used to determine the oxides present in the MSS. The oxides present in the adsorbent which may be attributed to adsorption study are shown in Table 2. The oxides of calcium, potassium, iron and phosphorus are the major constituents of the adsorbents indicating that the Mucuna pruriens seeds can be a source of obtaining useful elements. The FTIR analysis was used to examine the surface functional groups of the adsorbent and to identify those groups responsible for dye adsorption. The FTIR spectra of MSS and AMSS are shown in Figure 2 and Figure 3 (plot of IR transmittance against wave number). Their frequencies of the bands and the corresponding functional groups are presented in Table 3 and Table 4. The O-H stretch in alcohols which is a very strong and broad bond can be detected in the carbons which are important adsorption sites. The modifications in the frequencies of the bands present in MSS may be as a result of chemical activation and reactions with O-H groups. Some of the bands and intensities were shifted or removed and new peaks were also detected after reaction with the activating agent (H3PO4) indicating a change in the crystalline structure of the carbon (MSS). Xray diffraction (XRD) has been used to determine the diffractive pattern present on AMSS. The XRD spectrum of the carbons showed broad peaks (Figure 4), which indicates the presence of high content of amorphous form of carbon and little amounts of crystalline materials in the adsorbents. The three strongest peaks present in AMSS, with their interplanar spacings (d) and 2-Theta value (2θ) are presented in Table 5. The SEM micrographs of MSS and AMSS are presented as Figure 5 and Figure 6 respectively. Both SEM figures seem showed that H3PO4 activation increased the surface area of MSS. The SEM of AMSS (Figure 6) seems to be threadlike with, some breakage in nature and dotted illuminations.
3.2. Batch Adsorption Studies
3.2.1. Effect of Particle Size
The effect of particle size is shown in Figure 7. The percentage of CR and MG adsorbed decreased with increase in particle size of the adsorbents. This may be as a result of decreasing the particle size improves the pores available for adsorption.
3.2.2. Effect of Initial pH of Solution
Figure 8 shows that higher uptakes of CR and MG by AMSS was obtained at pH of 2 and 10 respectively. The reduction in adsorption capacity of CR dye on AMSS with increasing pH can be attributed to change in surface characteristics and charge. Also negatively charged surface site on the adsorbent does not favour the adsorption of anionic dye (CR) ions due to electrostatic repulsion and abundance of OH- ion [19] . The increase in the percentage adsorbed as the pH was increased may be caused by the electrostatic interaction between negatively charge adsorbent and cationic MG [5] . The best pH for each dye adsorption was used for further studies.
3.2.3 Effect of Adsorbent Dose
The percentage of the dyes adsorbed increased as the adsorbent dose was increased until saturation point was
![]()
Table 3. Fourier transform infrared spectrum for MSS.
![]()
Table 4. Fourier transform infrared spectrum for AMSS.
![]()
Table 5. Interplanar spacing, d of Xrays reflections for AMSS.
![]()
Figure 7. Effect of particle size on percentage adsorbed. Dosage = 1 g, Concentration = 100 mg/l, Time = 60 min, Temperature = 303 K.
reached (Figure 9). This may be as a result of increase in the number of active sites available for the process of adsorption which nearly becomes constant as the dosage was increased further This may be due to sites remaining unsaturated during the adsorption process [20] .
3.2.4. Effect of Adsorbate Concentration
Figure 10 depicts that the percentage removal of the textile dyes decreased as the adsorbate concentration was
![]()
Figure 8. Effect of initial pH of solution on percentage adsorbed. Particle size = 0.30 mm, Dosage = 1 g, Concentration = 100 mg/l, Time = 60 min, Temperature = 303 K.
![]()
Figure 9. Effect of adsorbent dose on percentage adsorbed. Particle size = 0.30 mm, pH (CR = 2, MG = 10), Concentration = 100 mg/l, Time = 60 min, Temperature = 303 K.
![]()
Figure 10. Effect of adsorbate concentration. Particle size = 0.30 mm, pH (CR = 2, MG = 10), Dosage = 1 g, Time = 60 min, Tem- perature = 303K.
increased. This may be at higher concentration, the number of dye ions competing for the available sites on the surface of adsorbent was high resulting in high percentage adsorbed [21] .
3.2.5. Effect of Contact Time
The percentage adsorbed increased with increase in contact time (Figure 11). The rate of removal of the textile dyes was rapid in the beginning due to the number of active sites available for the adsorption of dye ions and after a certain time, only a very low increment was observed.
3.3. Adsorption Isotherm
The equilibrium isotherm relationship between the concentration of the dyes in the liquid phase and the dyes in the adsorbent (AMSS) at a given temperature was studied. The results were analyzed using the Langmuir, Freundlich, Tempkin and Dubinin-Radushkevich isotherm.
![]()
Figure 11. Effect of contact time on percentage adsorbed. Particle size = 0.30 mm, pH (CR = 2, MG = 10), Dosage = 1 g, Concen- tration = 100 mg/l, Temperature = 303 K.
3.3.1. Langmuir Isotherm
The Langmuir model is expressed as [22] :
![]() (4)
(4)
Ce is the equilibrium concentration of dye (mg/l) and qe is the amount of the dye adsorbed (mg) per unit of activated carbon (g). Qm and KL are the Langmuir constants related to the adsorption capacity (mg/g) and the equilibrium constant (l/mg) respectively. The separation factor (RL) is a dimensionless constant used to predict whether an adsorption process is favourable or unfavourable which can be determined as follows [1] :
![]() (5)
(5)
where KL is a Langmuir constant. RL value implies the adsorption to be unfavourable (RL > 1), linear (RL = 1), favourable (0 < RL< 1), or irreversible (RL = 0). The plot of Ce/qe against Ce is shown in Figure 12. The correlation coefficients (R2) of 0.994 and 0.972 for CR and MG on AMSS respectively showed that the adsorption conformed to the Langmuir model. The separation factors, RL of 0.1632 and 0.0972 for CR and MG on AMSS respectively were found to be less than one indicating favourable adsorption (Table 6). The low SSE values of 0.747 and 1.071 for CR and MG on AMSS respectively indicates goodness of fit and adequacy of the model.
3.3.2. Freundlich Isotherm
Freundlich isotherm can be expressed as [16] [23] :
![]() (6)
(6)
This equation can be linearized as:
![]() (7)
(7)
The respective Freundlich constants, n and KF (Table 6) were calculated from the slopes and intercepts of the linear plots (Figure 13) of log qe versus log Ce. The values of KF (measure of adsorption) increased with increasing temperature. The R2 values of 0.980 and 0.999 for CR and MG on AMSS respectively obtained shows that the adsorption on AMSS conformed to the Frendlich isotherm generally. The low SSE values of 0.947 and 0.258 for CR and MG respectively on AMSS indicates goodness of fit and adequacy of the model.
3.3.3. Temkin Isotherm
The Temkin isotherm is expressed as [24] :
![]() (8)
(8)
![]()
Figure 12. Langmuir isotherm model on CR and MG adsorp- tion on AMSS.
![]()
Table 6. Calculated isotherm parameters for CR and MG adsorption on AMSS at 303 K.
The linear form of this isotherm can be given by
![]() (9)
(9)
qe is the amount adsorbed at equilibrium in mg/g; k1 is the Temkin isotherm energy constant. The constants bT which is related to the heat of adsorption and KT which is the equilibrium binding constant corresponding to the
![]()
Figure 13. Freundlich isotherm model on CR and MG adsorp- tion on AMSS.
maximum binding energy were determined from the plot of qe versus in Ce (Figure 14). The constants are presented in Table 6. The high values of bT of 230.06 and 226.95 J/mg for CR and MG on AMSS respectively indicate that the interaction between the adsorbate and the adsorbent was strong. The correlation coefficients, R2 value of 0.992 and 0.953 for CR and MG on AMSS respectively indicate that the isotherm model fitted well to the equilibrium adsorption experimental data. The low SSE values of 0.474 and 2.685 for CR and MG respectively on AMSS indicates goodness of fit and adequacy of the model.
3.3.4. Dubinin-Radushkevich Isotherm
The Dubinin-Radushkevich isotherm is given as [25] :
![]() (10)
(10)
![]() (11)
(11)
![]() (12)
(12)
where qD is the theoretical saturation capacity (mg/g), B is a constant related to mean free energy of adsorption per mole of the adsorbate (mol2/J2), ε is the polanyi potential, R is the universal gas constant (8.314 J/mol/K) and T is the temperature in Kelvin and E is the mean sorption energy. The D-R isotherm constants B, qe and E were obtained (Table 6) from the linear plots of Inqe against ε2 (Figure 15). The correlation coefficients, R2 of 0.810 and 0.775 for CR and MG on AMSS respectively obtained showed that the experimental data obtained did not fit well to the D–R isotherm (Table 6). The removal of CR and MG on AMSS is not a physical process since the values of mean free energy (E) obtained were found to be < 8 KJ/mol [26] . The low SSE values of 4.129 and 5.820 for CR and MG respectively on AMSS indicates that the other isotherm models fit the experimental data more than this model.
3.4. Kinetics of Adsorption
The adsorption kinetics was studied in order to study the mechanism of the process of adsorption.
3.4.1. Pseudo First-Order Kinetic Model
The pseudo-first-order Lagergren equation is given by [16] [21] :
![]() (13)
(13)
where qt and qe are the amounts of dye adsorbed at time t and equilibrium respectively and K1 (min−1) is the pseudo-first-order rate constant for the process of adsorption. K1 and qe were determined from the slope and intercept of the plot of log (qe − qt) versus t (Figure 16). The pseudo first order rate constants, K1 is given in Table 7. The values of the correlation coefficients (R2) of 0.928 and 0.959 for CR and MG respectively indicate that the removal of CR on AMSS did not follow the pseudo first-order kinetic model while MG removal on AMSS
![]()
Figure 14. Temkin isotherm model on CR and MG adsorption on AMSS.
![]()
Figure 15. Dubinin Ruduskevich isotherm model on CR and MG adsorption on AMSS.
![]()
Figure 16. Pseudo-first order kinetic model on CR and MG adsorption on AMSS.
follow the pseudo first-order kinetic model.
3.4.2. Pseudo Second-Order Kinetic Model
The pseudo second – order adsorption kinetic equation is expressed as [27] :
![]() (14)
(14)
k2 is the rate constant of pseudo second order adsorption (g/mg/min). The values of k2 and qe (Table 7) were calculated from the plots of t/qt versus t as shown in Figure 17. The R2 values which are close to unity (0.999) shows that the adsorption of CR and MG on AMSS is best described by the pseudo second-order mechanism suggesting that the rate-limiting step is a chemical adsorption [28] .
![]()
Figure 17. Pseudo-second order kinetic model on CR and MG adsorption on AMSS.
![]()
Table 7. Kinetic parameters and correlation coefficients obtained for the adsorption of CR and MG on AMSS at 303K.
3.4.3. Elovich Model
The Elovich kinetic model is expressed as [22] :
![]() (15)
(15)
Integration of this equation for the boundary conditions, gives:
![]() (16)
(16)
where α is the initial adsorption rate (mg/g min) and β is related to the extent of surface coverage and the activation energy for chemisorptions (g/mg). A plot of qt versus lnt yielded a linear relationship (Figure 18) where the Elovich constants, β and α were obtained and presented in Table 7. The adsorption of CR and MG on AMSS conformed to the Elovich kinetic model since the values of the linear regression coefficients (R2) are 0.982 and 0.979 respectively (Table 6).
![]()
Figure 18. Elovich kinetic model on CR and MG adsorption on AMSS.
3.4.4. Intraparticle Diffusion Kinetic Model
The intraparticle diffusion model equation is given by [29] :
![]() (17)
(17)
Where kpi is the rate parameter of stage i and Ci is the thickness of the boundary layer. The plot of qt versus t0.5 (Figure 19) gives a straight line. The intraparticle diffusion is not the only rate controlling step involved in the adsorption of CR and MG by the adsorbent since its linear plots did not pass through the origin [26] .
3.5. Thermodynamics of Adsorption
The activation energy was evaluated from the pseudo second-order kinetic rate constants obtained from adsorptive removals performed at 303, 313 and 323K. The Arrhenius equation is given as [30] :
![]() (18)
(18)
where k2 is the rate constant obtained from the pseudo second-order kinetic model (g/mg h), Ea is the Arrhenius activation energy of adsorption (kJ/mol) and A is the Arrhenius factor. In k2 was plotted against 1/T and Ea was obtained from the slope. The activation energies of adsorption of CR on AMSS is 19.9182 K/Jmol which implies that the rate-limiting step might be a physically controlled process since which is less than 40 K/Jmol and MG adsorption on AMSS is 66.7789 K/Jmol which is greater than 40 K/Jmol, might be a chemically controlled process [31] .
Adsorption thermodynamics is the study of the effect of temperature on the process of adsorption. ∆G0 and ∆H0 were used to evaluate the spontaneity and nature of the adsorption process respectively [28] . The thermodynamic parameters free energy (∆G0), enthalpy (∆H0) and entropy (∆S0) were determined as follows [32] :
![]() (19)
(19)
![]() (20)
(20)
where R (8.314 J/mol K) is the universal gas constant, T (K) is the solution temperature and KL (L/mg) is the Langmuir isotherm constant. The values of ΔH0 and ΔS0 were calculated, respectively from the slope and intercept of the Vant Hoff plot of ln KC versus 1/T (Figure 20) are presented in Table 8. The values of ∆G0 were found to be negative which decreased with increased temperature showing that the adsorption was more favourable and spontaneous at higher temperature (Table 8). The positive value of ∆H0 indicates the endothermic nature of the process. The positive value of ∆S0 indicates an increase in the randomness at the solid/solution interface due to the redistribution of energy between dyes and the adsorbents [23] . The removal of MG was found to be more spontaneous than the removal of CR on AMSS.
4. Conclusion
Activated carbon prepared from Mucuna pruriens seeds shells (abundantly available agricultural waste) using
![]()
Figure 19. Intraparticle diffusion model on CR and MG adso- rption on AMSS.
![]()
Figure 20. Vant Hoff plot on CR and MG adsorption on AMSS.
![]()
Table 8. Thermodynamic parameters for the adsorption of CR and MG dye on AMSS.
Particle size = 0.3 mm, Contact time = 60 min, Initial concentration = 100 mg/l and adsorbent dose = 1 g.
chemical activation was used for the removal of congo red and malachite green (textile dyes) from aqueous solution. The removal efficiencies of using H3PO4 activated carbon (AMSS) on CR and MG were compared. The batch adsorption studies indicated that the percentage adsorbed depended on particle size, adsorbent dose, pH, contact time and adsorbate concentration. The adsorption of AMSS on CR and MG was found to follow the Langmuir, Freundlich and Tempkin isotherms. The pseudo second order kinetic was found to best correlate the experimental data obtained. The intraparticle diffusion is involved in the adsorption of CR and MG by the adsorbent but it is not the only rate controlling step. The negative free energy change (∆G0) and positive value of entropy (ΔS0) indicate the feasible and spontaneous nature of the process. The removal of MG was found to be more spontaneous and feasible than the removal of CR on AMSS. AMSS was found to be a better adsorbent for MG removal than for CR. The thermodynamic and kinetics data can be further used for the design of a plant for treatment of industrial wastewater containing congo red and malachite green on large scale.
Acknowledgements
Authors are grateful to the Faculty of Engineering, Nnamdi Azikiwe University, Awka, Nigeria; National Research Institute for Chemical Technology (NARICT), Zaria, Nigeria; National Metallurgical Training Institute, Onitsha, Nigeria; and Scientific Equipment Development Institute (SEDI), Enugu, Nigeria.
NOTES
![]()
*Corresponding author.