Composites on Base of the Ultradispersed Polytetrafluoroethylene and Graphite Oxide Intercalated Compounds ()


1. Introduction
Polytetrafluoroethylene (PTFE) is one of the most thoroughly studied polymers that has found rather extensive practical applications [1] [2]. In particular, PTFE-M (M-Al, Mg, Ti, Zr etc.) composites were suggested as mixed energy-rich powders [3]. They are produced using the method of combined grinding in ball mills. The phenomenon of transition of heavy polymer molecules into the gas phase in the process of PTFE thermal destruction with subsequent homophase nucleation and condensation in the form of nanofilms of a thickness of 2 - 10 nm was discovered for the first time in the Institute of Chemistry FEBRAS in 1982. Depending on the condensation conditions, nanofilms can grow up to sizes of 100 × 100 µm and roll up into microtubes of a length of up to 150 µm or a size of about 2 × 2 µm with subsequent formation of a pack of nanofilms as a microsphere of a diameter of about 1 µm [4]. This material comprising a mixture of PTFE oligomers with the polymer chain length containing from ≈101 to 103 CF2-groups was named ultradispersed polytetrafluoroethylene (UPTFE) [5]. A significant difference between UPTFE and PTFE (in the form of fluoroplast-4) is related to their powders particle sizes: 0.1 - 3.0 µm and 160 - 200 µm, respectively. The latter fact opened new possible fields of practical application of UPTFE or significantly improved the available ones [6]. Equally important in fundamental and applied aspects is the graphite oxide (GО) [7] [8]. For example, fabrication of composites based on UPTFE and intercalated GO compounds with dodecahydro-closo-dodecaborate acid (Н2В12Н12), which are rather promising as energy-rich materials, can be performed through ultrasonic treatment of components aqueous solutions without the pre-pounding of fluoropolymer [9]. This is simpler and safer than application of mechanochemical methods [3]. In these composites, Н2В12Н12 serves as a combustible, while combined UPTFE and GO―as an oxidizer. As fuel can be not only Н2В12Н12, and its salts [10] [11].
The objective of the present work was to develop composites of UPTFE and IC of GO with Н2В12Н12 or (NН4)2В12Н12 and methods of their synthesis and study their properties.
2. Experimental
Studies of the process of thermal destruction of PTFE (fluoroplast-4) yielded the development of the thermal gas-dynamic (TGD) method and the technology of UPTFE production capable to create UPTFE manufacture on an industrial scale [12].
GO was obtained via the oxidation of natural graphite from the Zaval’ev deposit (Ukraine) using the modified Hummers-Offeman approach described in detail in [13]. After sedimentation of the reaction solution, the layer of clarified solution was decanted from the GO gel, and a fresh portion of distilled water was poured onto the sediment. After bringing the solution pH to 1 - 2, where the GO gel sedimentation rate dropped dramatically and the interface became diffuse, the solution was centrifuged on a K 70D centrifuge (Germany) at 7000 rpm. The GO gel was washed to pH ≈ 5 finally. To determine the content of the “dried” GO sample of his water-based gel was dried as a thin film at room temperature, and then milled into a powder and finely dried at 100˚C until constant weight. To prevent the GO photoreduction a time-consuming operation of washing and drying the GO carried out in the dark. Analysis of “dried” GO into carbon and hydrogen was carried out by known methods of microanalysis of organic substances [14]. The composition of “dried” GO corresponds to the empirical formula С6О2,40Н1,32 × 0.58 Н2О.
Potassium dodecahydro-closo-dodecaborate (К2В12Н12) was obtained by pyrolysis of KBH4-MBF4 mixture (М-Na, K) according to [15]. The В12Н122−-anion separation from reaction products and its purification were performed using chitosan [16]. Salts of the В12Н122−-anion were obtained by treatment of chitosan dodecahydro- closo-dodecaborate (С6О4Н9NH3)2В12Н12 by respective hydroxides. To obtain the acid Н2В12Н12, the cation exchange process of М2В12Н12 salts (M-Na) on the KU-2 ion exchange resin was used.
The content of the В12Н122−-anion in solutions of acid and ammonium salt was determined using the gravimetric method as Ag2В12Н12 [17].
IC synthesis was carried out through interaction of an aqueous gel of GO with aqueous solutions of Н2В12Н12 and (NН4)2В12Н12 at a molar ratio of 1 to (0.1 - 0.3) [10] [11].
Optical images of the composite films were obtained with a microscope Axioplan 2 (Karl Zeiss, Germany).
The X-ray diffraction analysis was carried out on a DRON-3 and a D8 ADVANCE diffractometers (Bragg-Brentano method, λCuKa).
3. Results and Discussion
In the course of development of energy-active composites UPTFE-GO × nM2B12H12 (M-H, NН4), i.e., those that decompose the most completely, the following points were taken into consideration.
First, as was established earlier [18], heating of IC GO × nН2В12Н12 induced the exothermic reaction of intramolecular oxidation of dodecahydro-closo-dodecaborate fragments by oxygen present in the structure of GO. Here, the result is the formation of partially oxidized closo-dodecaborate anions B12H10О2− self-organizing into the boron-polymer material [-B12H10О2−-]n and the carbonaceous residue formed from the residual GO. ICs with high content of M2B12H12 (M-Н, Li, Na, K etc.) [19] were recommended as film-forming agents of water-based dispersion paints, whose drying yields thin coatings with high adhesion to the surface insoluble in water and a majority of known organic solvents and stable at heating in air up to 300˚C. It was demonstrated that at low M2В12Н12 content in IC, namely for compositions GO × nM2В12Н12 (n ≤ 0.3), the amount of oxygen in the structure of GO was sufficient for more complete oxidation of the B12H122−-component. Heat emitted in this process warms up intermediate decomposition products up to temperatures sufficient for involving air oxygen into their complete oxidation. Decomposition of such ICs could proceed as an explosion or a flash upon fast heating or mechanical impact as in an inert atmosphere as in air [9] [10] [18].
Second, in development of such composites, one should take into account the ratio between UPTFE and IC. The ratio must ensure complete transformation of boron into boron oxyfluoride in air. It can be calculated from the following schemes:
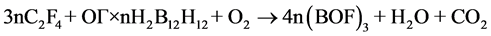 (1)
(1)
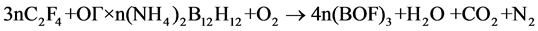 (2)
(2)
The calculations show that in the case of UPTFE-GO × nН2В12Н12 composites the fluoropolymer content must be equal to 19.6 (n = 0.1), 30.4 (n = 0.2), and 37.2 wt% (n = 0.3). In the case of UPTFE-GO × n(NН4)2В12Н12 composites, this value must be equal to 19.2 (n = 0.1), 29.4 (n = 0.2), and 35.7 wt% (n = 0.3). At the М2В12Н12 content increase in the composite, larger quantities of energy are emitted as a result of primary reaction of intramolecular interaction in IC GO. The latter results in more intensive heating of intermediate decomposition products that is sufficient for involvement of air oxygen and UPTFE into the process of complete oxidation. On the other hand, if the M2В12Н12 content increases, one has to increase the fraction of UPTFE in the composite in order to transform boron into boron oxyfluoride. In this case, the mass of products heated during primary interaction increases, so that the emitted heat could be insufficient to warm up intermediate decomposition products up to temperatures sufficient for involvement of air oxygen with attainment of complete combustion.
To sum up, to fabricate energy-active composites, it is necessary to determine experimentally the optimal ratio between their components (UPTFE, GO, and M2B12H12), since respective calculations are rather complicated due to lack of thermodynamic data.
As was found earlier, to fabricate UPTFE-GO × nH2В12Н12 composites [9], an intensive physical impact on the mixture of GO and UPTFE was used. It is related to the fact that UPTFE is characterized by expressed hydrophobic properties, and its introduction into the solution bulk is a very complicated task that is realized through powerful mechanical impact on UPTFE particles using two methods. The first one is concerned with using a high-speed stirrer (20,000 rpm), the second one―with ultrasonic impact. For instance, ingress of hydrophobic UPTFE particles into a narrow gap between rotating and stationary cylindrical shell rings of the stirrer results in their gradual activation due to emergence of dislocations, vacancies, shifts, and other defects in the polymer structure. As a result, particles become hydrophilic and move from the surface into the bulk of the GO aqueous gel. Similar modifications of UPTFE particles occur under effect of mixtures ultrasonic treatment as well.
The task can be significantly simplified by using ethanol dispersions of UPTFE, in which fluoropolymer particles are completely wetted by alcohol and do not float on its surface. Here, it was necessary to examine whether mixing of IC GO gel with UPTFE dispersion will result in gel destruction or M2В12Н12 salting-out from the IC GO composition.
As was established by the performed studies, mixing the aqueous gel of IC with the alcohol dispersion of UPTFE with the fluoropolymer content in the composite up to 40 wt% yielded the formation of homogeneous non-exfoliating gels. Upon their concentrating in air occurring mainly at the expense of ethanol evaporation and subsequent drying until the solid state, one does not observe chipping of UPTFE particles from the composite. According to the optical microscopy data, UPTFE particles are randomly distributed in the homogeneous film of IC (Figure 1). Composites diffractograms show the presence of PTFE and IC GO in them (Figure 2), which corroborates the absence of salting-out effect of ethanol on acid or ammonium salt and IC destruction. Upon ignition in air, all the fabricated composites explode or burn actively with formation of a light sooty residue. When conditioning in a humid atmosphere the weight gain of samples of the composite is not observed.
To sum up, composites based on UPTFE with intercalated compounds of GO with M2B12H12 (M-Н, NН4) have been developed. The optimal ratio between all the composite components has been found. A simple method of composite fabrication has been developed. The composites can be used as rather promising components of energy-rich materials of different functional applications.
![]()
Figure 1. Optical image of the composite UPTFE-GO × 0.3H2B12H12.
![]()
Figure 2. XRD of composite UPTFE-GO × 0.2(NН4)2B12H12.
Acknowledgements
The work financially supported by the Program of Basic Research “”Far East” (project no. 265-2015-0011).