Design and Synthesis of Some Enaminonitrile Derivatives of Antipyrine as Potential Novel Anti-Inflammatory and Analgesic Agents ()
1. Introduction
Enaminonitriles are important intermediates for the preparation of heterocyclic compounds possessing diverse biological activities. They are of particular interest as very promising reagents for cascade heterocyclization, which will undoubtedly become one of the main approaches to the targeted synthesis of heterocycles in the near future, in the rapidly-rising field of combinatorial chemistry [1] -[3] . Non-steroidal anti-inflammatory drugs (NSAIDs) represent a heterogeneous family of pharmacologically active compounds used to alleviate acute and chronic inflammation, pain and fever.
Their clinical efficacy is closely related to their ability to inhibit both COX-1 and COX-2 isoforms of the enzyme cyclooxygenase (COX); it also referred to prostaglandin H2 synthase since it catalyzes the conversion of arachidonic acid to prostaglandin H2 (PGH2) [4] . The constitutive COX-1 isoform is mainly responsible for the synthesis of prostaglandins which exert cytoprotective effect on the gastrointestinal (GI) tract and control renal function in the kidneys, whereas, the inducible COX-2 is selectively activated by pro-inflammatory stimuli and facilitates the release of prostaglandins involved in the inflammatory process [5] . Consequently, their long-term clinical employment is associated with significant side effects such as gastrointestinal lesions, bleeding, and nephrotoxicity [6] . Since the introduction of antipyrine; the first pyrazolone derivative used in the management of pain, inflammation and fever into clinical use in 1884, great attention has been focused on pyrazole derivatives as potent anti-inflammatory, analgesic and antipyretic agents [7] . As a result, a large number of pyrazoles have been obtained and gained application on the clinical level.
Furthermore, diverse chemotherapeutic activities have been ascribed to pyrazoles as antimicrobial [8] , antiparasitic [9] , antiviral [10] and antineoplastic agents [11] . Interest in this field has been intensified after the discovery of the natural pyrazole C-glycoside pyrazofurin; 4-hydroxy-3-b-D-ribofuranosyl-1H-pyrazole-5-carboxa- mide. This antibiotic was reported to possess a broad spectrum of antimicrobial and antiviral activities in addition to being active against several tumor cell lines [12] . On the other hand, careful literature survey revealed that thiazole ring system has occupied a unique position in the design and synthesis of novel biologically active agents with remarkable analgesic and anti-inflammatory activities, in addition to their well documented potential antimicrobial activities [13] [14] .
It was of interest to study the reactivity of antipyrinylhydrazono-malononitrile towards different nitrogen nucleophiles as well as activated nitriles. In continuation of our studies on the chemistry of enamino and activated nitriles [15] - [26] and as a part of our program directed toward developing new approaches to a variety of heterocycles incorporating the antipyrine moiety [15] [16] of potential biological activity, we report here the scope and applicability of 2-[(1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)-hydrazono] malononitrile as a unique precursor for the syntheses of novel acyclic enaminonitriles in which a antipyrine ring is incorporated.
2. Results and Discussion
2.1. Chemistry
The synthetic strategies adopted to obtain the target compounds are depicted in Scheme 1. The starting (1,5- dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)carbonohydrazonoyl di-cyanide (2) [27] was prepared by diazotization of 4-aminoantipyrine (1) and coupling with malononitrile in ethanolic sodium acetate solution at 0˚C - 5˚C. Compound 2 reacted with different secondary amines namely; [piperidine, morpholine, N-methylgl- ucamine, pyrrolidine, diphenyl amine, ethyl 2-(4-chlorophenylamino)acetate, piperazine and 1-phenylpiperazine] in refluxing ethanol to afford the corresponding 1:1 acyclic enaminonitrile adducts 3-10, respectively. The formation of enaminonitriles 3-10 was illustrated through the initial addition of the secondary amines to cyano function to form the imino form followed by [1,5]H migration to form the enamine form (Scheme 1). The struc- tures of enaminonitriles 3-10 were confirmed by elemental analyses and spectral data. The IR spectra exhibited absorption bands due to stretching vibrations of NH2 group within υ = 3450 - 3301 cm−1, within υ = 2186 - 2171 cm−1 due to CN function and within υ = 1648 - 1610 cm−1 due to carbonyl groups. The 1H-NMR spectrum of compound 3 revealed the presence of three multiplet signals at δ 1.58 - 1.69, 3.52 - 3.62 and 7.31 - 7.52 ppm attributable to (3CH2, piperidine), (2CH2, piperidine) and aromatic protons, respectively, revealed two singlet signals at δ 2.63 and 3.16 ppm due to methyl and N-methyl protons, respectively and amino protons appeared at δ 7.13 ppm as broad singlet signal. The 13C-NMR spectra revealed signals due to cyano group within δ = 114.8 - 114.3 ppm. Furthermore, the detailed 1H- and 13C-NMR spectra for each compound were present in the experimental section. Moreover, the mass spectroscopic measurement of compounds 3-5 and 8-10 showed the molecular ion peaks at 367 (M+, 12.3), 368 (M+-1, 6.7), 477 (M+, 100.0), 495 (M+, 17.5), 368 (M+, 11.4) and 444 (M+, 5.0), respectively, which are equivalent with the molecular formula of the structures.
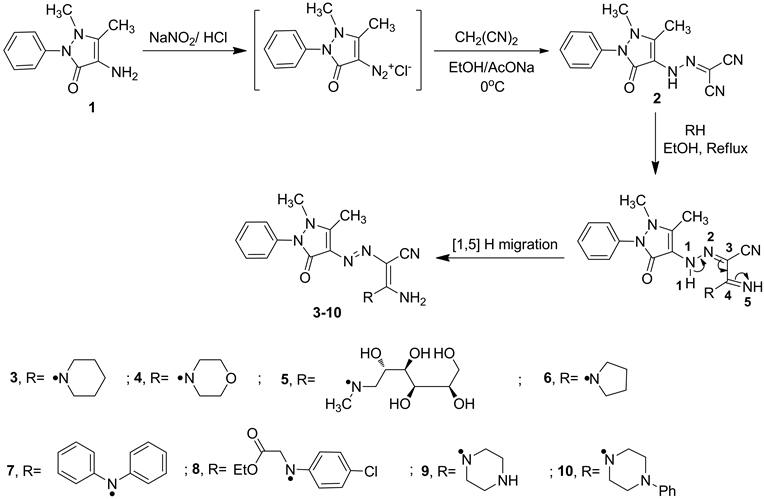
Scheme 1. Synthetic route for the preparation of acyclic enaminonitriles 3-10.
2.2. Pharmacology
2.2.1. Anti-Inflammatory Activity
The anti-inflammatory activity was evaluated by the carrageenan-induced paw edema test on rats. The anti- inflammatory activity data (Table 1) indicated that all the tested compounds protected rats from carrageenan- induced inflammation, and tested compounds (3, 4, 9 and 10) are the most potent among tested compounds. Compounds 3, 4, 9, and 10 showed similar and or equivalent anti-inflammatory activity when compared to diclofenac sodium.
2.2.2. Analgesic Activity
The analgesic activity was determined by the hot plate test (central analgesic activity) and acetic-acid induced writhing assay. The results from in Table 2 & Table 3 revealed that compounds 3, 4, 9, and 10 exhibited significant activity. Most of the tested compounds have analgesic activity. Compound 10 exhibited comparable effect relative to the reference drug in peripheral analgesic activity testing.
Subsequently, we may conclude the following structure activity relationship’s (SAR’s). 1) The presence of basic skeleton (piperidyl, morpholino and piperazinyl moieties) is necessary for the broad spectrum of anti- inflammatory and analgesic activities (carrageenan induced paw odema test in rats, writhing assay and hot plate test). 2) Introducing a amino group (electron donating group) in aminoantipyrine (1) decreases the activity. 3) Introducing of five hydroxyl groups in the basic skeleton of compound 5 increases the anti-inflammatory activity but decreases the analgesic activity using different methods. 4) Introducing of ester group in compound 8 decreases the anti-inflammatory and analgesic activities, but only increase the analgesic activity in case of using hot plate test. 5) According to the above findings the presence of basic skeleton of six-membered rings enhanced anti-inflammatory and analgesic activities (compounds 3, 4, 9 and 10). 6) In compound 7 the presence of two phenyl moieties beside the other phenyl ring in the basic skeleton of antipyrine increases the anti-inflammatory and analgesic activities. 7) Compound 6 has a basic pyrrolidinyl moiety (five-membered ring) enhance the activities, showed weak analgesic activity using writhing test. 8) In general, aminoantipyrine (1) showed weak anti- inflammatory and analgesic activities (Figure 1).
3. Conclusion
Newly synthesized antipyrine compounds seem to be interesting for biological activity studies. Furthermore, the
![]()
Table 1. Percent anti-inflammatory activity of the tested compounds (carrageenan induced paw odema test in rats).
Each value represents the mean ± S.E (n = 6). Significance levels *p < 0.5, **p < 0.001 as compared with respective control. Dose (20 mg/kg). For the selected tested compound.
![]()
Table 2. Central analgesic activity (hot plate test).
Values represent the mean ± S.E. of six animals for each groups. ap < 0.05: Statistically significant from Control. (Dunnett’s test); bp < 0.05: Statistically significant from ASA. (Dunnett’s test). *Significant at p < 0.05.
![]()
Table 3. Percent analgesic activity (peripheral, writhing test).
Each value represents the mean ± S.E (n = 6). Significance levels *p < 0.5, **p < 0.001 as compared with respective control. Dose (20 mg/kg). For the selected tested compound.
![]()
Figure 1. Structure activity relationship’s (SAR’s) of the more potent anti-inflammatory and analgesic compounds.
present investigation offers rapid and effective new procedures for the synthesis of novel enaminonitriles incorporated antipyrine moiety. It is worth mentioning that the incorporation of six membered ring containing hetero atoms into enaminonitriles of antipyrine compounds was crucial for the anti-inflammatory and analgesic activities as in the case of compounds 3, 4, 9 and 10.
4. Experimental
4.1. Synthesis
All melting points are recorded on Gallenkamp electric melting point apparatus. The IR spectra υ cm−1 (KBr) were on Perkin Elmer Infrared Spectrophotometer Model 157, Grating. The 13C-NMR and 1H-NMR spectra were run on Varian Spectrophotometer at 100 and 400 MHz, respectively, using tetramethylsilane (TMS) as an internal reference and using dimethylsulfoxide (DMSO-d6) as solvent. The mass spectra (EI) were run at 70 eV with JEOL JMS600 equipment and/or a Varian MAT 311 A Spectrometer. Elemental analyses (C, H and N) were carried out at the Microanalytical Center of Cairo University, Giza, Egypt. The results were found to be in good agreement with the calculated values. Antipyrine (mp 110˚C - 113˚C) and 4-aminoantipyrine (1) (mp 106˚C - 110˚C) were purchased from Aldrich Company. (1,5-Dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol- 4-yl)carbonohydrazonoyl dicyanide (2) was prepared according to the previously reported method [27] (93%), mp 140˚C; yellowish orange crystals; 1H-NMR (400 MHz, DMSO-d6): δ, 2.26 (s, 3H, CH3), 3.25 (s, 3H, N-CH3), 7.35 - 7.56 (m, 5H, Ph), 12.1 (br., s, 1H, NH); MS: (m/z, %): 281 (M+ + 1, 4.3), 280 (M+, 13.4), 188 (5.2), 91 (8.1), 56 (100.0).
General procedure for the synthesis of 3-amino-2-(1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol- 4-ylazo)-[3-substituted]-1-yl-acrylonitrile derivatives 3-10.
A mixture of compound 2 (1.4 g, 5 mmol) and the appropriate secondary amine namely; piperidine (0.49 mL, 5 mmol), morpholine (0.43 mL, 5 mmol), N-methylglucamine (0.98 g, 5 mmol), pyrrolidine (0.41 mL, 5 mmol), diphenyl amine (0.85 g, 5 mmol), ethyl 2-(4-chlorophenylamino)acetate (1.07 g, 5 mmol), piperazine (0.43 g, 5 mmol) or 1-phenylpiperazine (0.81 g, 5 mmol) in ethanol (15 mL) was refluxed for 5 h. The reaction mixture was left to cool and the precipitated solid was filtered off, dried and recrystallized from EtOH/DMF (2:1) mixture to afford the corresponding acyclic enaminonitrile derivatives 3-10, respectively.
3-Amino-2-(1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-ylazo)-3-piperidin-1-yl-acrylonitrile (3). Yield (91%), mp 209˚C; dark green crystals; IR (KBr) ύ (cm−1), 3392, 3334 (NH2), 3189 (NH), 2960 (C-H, stretching), 2171 (CN), 1639 (CO), 1448 (N=N); 1H-NMR (400 MHz, DMSO-d6): δ, 1.58 - 1.69 (m, 6H, 3CH2, piperidine), 2.63 (s, 3H, CH3), 3.16 (s, 3H, N-CH3), 3.52 - 3.62 (m, 4H, 2CH2, piperidine), 7.13 (br., s, 2H, NH2), 7.31 - 7.52 ppm (m, 5H, Ph); 13C-NMR (100 MHz, DMSO-d6): δ, 183.2, 160.4, 160.1, 136.6, 136.5, 129.1, 129.0, 119.7, 119.5, 114.8, 113.2, 113.1, 113.0, 95.7, 46.8, 46.2, 46.1, 39.8, 25.9, 25.8, 25.7, 13.1 ppm. MS: (m/z, %): 367 (M+, 12.3), 366 (M+-1, 14.5), 338 (12.2), 280 (11.0), 215 (11.0), 189 (77.9), 152 (100.0), 86 (12.8), 63 (26.7). Anal. for C19H25N7O (367.45): Calcd. C, 62.10; H, 6.86; N, 26.68%; Found: C, 62.23; H, 6.91; N, 26.76%.
3-Amino-2-(1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-ylazo)-3-morpholin-4-yl-acrylonitrile (4). Yield (83%), mp 232˚C; light brown crystals; IR (KBr) ύ (cm−1), 3385, 3337 (NH2), 3197 (NH), 2967 (C-H, stretching), 2186 (CN), 1637 (CO), 1470 (N=N); 1H-NMR (400 MHz, DMSO-d6): δ, 2.22 - 2.25 (m, 4H, 2CH2, morpholine), 2.44 (s, 3H, CH3), 3.10 (s, 3H, N-CH3), 3.58 - 3.74 (m, 4H, 2CH2, morpholine), 7.24 (br., s, 2H, NH2), 7.36 - 7.51 ppm (m, 5H, Ph); 13C-NMR (100 MHz, DMSO-d6): δ, 183.2, 160.5, 160.3, 136.6, 136.5, 129.4, 129.1, 119.7, 119.5, 114.8, 113.3, 113.1, 113.0, 95.7, 67.2, 64.9, 47.1, 46.8, 39.8, 13.1 ppm. MS: (m/z, %): 368 (M+-1, 6.7), 367 (M+-2, 15.5), 275 (7.7), 214 (13.4), 188 (14.6), 108 (24.6), 96 (17.8), 56 (100.0); Anal. for C18H23N7O2 (369.42): Calcd. C, 58.52; H, 6.28; N, 26.54%; Found: C, 58.61; H, 6.33; N, 26.61%.
3-Amino-2-(1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-ylazo)-3-[methyl-(2,3,4, 5,6-penta-
hydroxy-hexyl)-amino]-acrylonitrile (5). Yield (83%), mp 205˚C; dark yellow crystals; IR (KBr) ύ (cm−1), 3451, 3436 (OH), 3358, 3301 (NH2), 2954 (C-H, stretching), 2186 (CN), 1648 (CO), 1459 (N=N); 1H-NMR (400 MHz, DMSO-d6): δ, 2.47 (s, 3H, CH3), 3.16 (s, 3H, N-CH3), 3.35 - 3.41 (m, 5H, CH2-N-CH3), 3.86 - 3.93 (m, 2H, CH2O), 4.36-5.14 (br, m, 5H, 5OH), 7.33 (br., s, 2H, NH2), 7.35 - 7.53 ppm (m, 5H, Ph); 13C-NMR (100 MHz, DMSO-d6): δ, 183.3, 160.6, 160.1, 136.6, 136.5, 129.1, 129.3, 119.8, 119.5, 114.8, 113.5, 113.1, 113.1, 95.7, 72.9, 72.8, 71.6, 71.3, 70.8, 64.9, 51.6, 46.8, 39.8, 35.9, 13.2 ppm. MS: (m/z, %): 477 (M+, 100.0), 438 (97.0), 282 (78.8), 279 (48.5), 241 (93.9), 178 (69.7), 163 (57.6), 144 (63.6), 104 (45.5), 94 (15.2), 57 (30.3); Anal. for C21H31N7O6 (477.51): Calcd. C, 52.82; H, 6.54; N, 20.53%; Found: C, 52.91; H, 6.59; N, 20.72%.
3-Amino-2-(1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-ylazo)-3-pyrrolidin-1-yl-acrylonitrile (6). Yield (88%), mp 229˚C; light brown sheets; IR (KBr) ύ (cm−1), 3367, 3272 (NH2), 3183 (NH), 2944, 2875 (C-H, aliphatic), 2173 (CN), 1641 (CO), 1467 (N=N); 1H-NMR (400 MHz, DMSO-d6): δ, 1.92 - 2.09 (m, 4H, 2CH2, pyrrolidine), 2.44 (s, 3H, CH3), 3.10 (s, 3H, N-CH3), 3.50 - 3.69 (m, 4H, 2CH2, pyrrolidine), 6.73 (br., s, 2H, NH2), 7.31 - 7.51 ppm (m, 5H, Ph); 13C-NMR (100 MHz, DMSO-d6): δ, 183.3, 160.5, 160.1, 136.8, 136.5, 129.1, 129.0, 119.7, 119.6, 114.8, 113.4, 113.1, 113.0, 95.8, 49.5, 49.6, 26.2, 26.1, 26.0, 46.8, 39.8, 13.1 ppm. Anal. for C18H23N7O (353.42): Calcd.: C, 61.17; H, 6.56; N, 27.74%; Found: C, 61.26; H, 6.61; N, 27.83%.
3-Amino-2-(1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-ylazo)-3-diphenylamino acrylonitrile
(7). Yield (75%), mp 98˚C; light black powder; IR (KBr) ύ (cm−1), 3352, 3271 (NH2), 2179 (CN), 1644 (CO), 1472 (N=N); 1H-NMR (400 MHz, DMSO-d6): δ, 2.42 (s, 3H, CH3), 3.18 (s, 3H, N-CH3), 6.63 - 7.54 (m, 15H, Ar-H), 8.14 ppm (br., s, 2H, NH2); 13C-NMR (100 MHz, DMSO-d6): δ, 183.4, 160.4, 160.1, 140.8, 136.7, 136.5, 129.8, 129.7, 129.6, 129.2, 129.0, 119.7, 119.6, 119.2, 119.1, 118.7, 118.7, 118.6, 118.4, 114.8, 113.4, 113.1, 113.2, 95.7, 46.9, 39.8, 13.3 ppm. Anal. for C26H25N7O (451.52): Calcd.: C, 69.16; H, 5.58; N, 21.71%; Found: C, 69.27; H, 5.63; N, 21.79%.
[1-Amino-2-cyano-2-(1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-ylazo)-vinyl]-(4-chloro-phenyl)-amino]-acetic acid ethyl ester (8). Yield (75%), mp 88˚C - 90˚C; light black powder; IR (KBr) ύ (cm−1), 3358, 3266 (NH2), 2183 (CN), 1740 (C=O, ester), 1648 (CO), 1479 (N=N); 1H-NMR (400 MHz, DMSO-d6): δ, 1.29 (t, 3H, CH2CH3, J = 7.2 Hz), 2.41 (s, 3H, CH3), 3.18 (s, 3H, N-CH3), 3.82 (s, 2H, CH2), 4.12 (q, 2H, CH2CH3, J= 7.2 Hz), 6.2 (br, s, 2H, NH2), 7.01 - 8.12 (m, 9H, Ar-H); 13C-NMR (100 MHz, DMSO-d6): δ, 183.2, 169.5, 160.5, 160.1, 142.3, 136.6, 136.5, 129.3, 129.1, 129.0, 122.8, 119.7, 119.6, 115.2, 115.3, 114.8, 113.3, 113.1, 113.0, 95.7, 62.1, 50.3, 46.8, 39.8, 14.8, 13.1. MS: (m/z, %): 495 (M+, 17.5), 447 (0.2), 214 (7.5), 212 (19.6), 141 (33.0), 139 (100.0), 56 (16.0); Anal. for C24H26ClN7O3 (495.96): Calcd.: C, 58.12; H, 5.28; N, 19.77%; Found: C, 58.21; H, 5.34; N, 19.81%.
3-Amino-2-(1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-ylazo)-3-piperazin-1-yl-acrylonitrile
(9). Yield (72%), mp 89˚C - 90˚C; dark red powder; IR (KBr) ύ (cm−1), 3450, 3379 (NH2), 3159 (NH), 2929 (C-H, stretching), 2174 (CN), 1639 (CO), 1494 (N=N); 13C-NMR (100 MHz, DMSO-d6): δ, 183.3, 160.4, 160.1, 136.7, 136.5, 129.1, 129.0, 119.7, 119.5, 114.8, 113.3, 113.1, 113.0, 95.7, 50.6, 50.5, 46.8, 46.9, 46.6, 39.8, 13.1. MS: (m/z, %): 368 (M+, 11.4), 343 (1.0), 228 (2.9), 201 (6.9), 189 (10.0), 160 (17.5), 135 (69.5), 73 (100.0), 65 (20.8); Anal. for C18H24N8O (368.44): Calcd.: C, 58.68; H, 6.57; N, 30.41%; Found: C, 58.63; H, 6.51; N, 30.38%.
3-Amino-2-(1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-ylazo)-3-(4-phenyl piperazin-1-yl)- acrylonitrile (10). Yield (86%), mp 230˚C; yellow powder; IR (KBr) ύ (cm−1), 3390, 3334 (NH2), 2925, 2809 (C-H, aliphatic), 2173 (CN), 1610 (CO), 1490 (N=N); 1H-NMR (400 MHz, DMSO-d6): δ, 2.44 (s, 3H, CH3), 3.10 (s, 3H, N-CH3), 3.28 - 3.36 (m, 4H, 2CH2, piperazine), 3.72 - 3.82 (m, 4H, 2CH2, piperazine), 6.12 (br., s, 2H, NH2), 6.81 - 7.53 (m, 5H, Ph); 13C-NMR (100 MHz, DMSO-d6): δ, 183.2, 160.4, 160.1, 149.7, 136.6, 136.5, 130.2, 130.1, 129.1, 129.0, 119.7, 119.5, 118.4, 114.8, 114.4, 114.3, 113.2, 113.1, 113.0, 95.7, 50.7, 50.6, 47.3, 46.8, 39.8, 13.1. MS: (m/z, %): 444 (M+, 5.0), 375 (0.4), 228 (46.6), 214 (65.3), 188 (82.4), 162 (59.7), 132 (94.7), 120 (100.0), 99 (67.3), 88 (42.7), 73 (81.9), 66 (24.3); Anal. for C24H28N8O (444.53): Calcd.: C, 64.84; H, 6.35; N, 25.21%; Found: C, 64.92; H, 6.39; N, 25.27%.
4.2. Material and Methods
4.2.1. Animals
Female Sprague-Dawley rats (150 - 200 g) were used in the study of anti-inflammatory activity [28] [29] . Both sex of Swiss mice weighing (25 - 30 gm) used in analgesic activity and, taking into account international principle and localregulations concerning the care and use of laboratory animals [30] . The animals had free access to standard commercial diet and water ad libitum and were kept in rooms maintained at 22˚C ± 1˚C with 12 h light dark cycle.
4.2.2. Anti-Inflammatory Activity (Carrageenan-Induced Rat Hind Paw Edema Model)
The method adopted resembles essentially described by Winter et al. [31] , (distilled water) was selected as vehicle to suspend the standard drugs and the test compounds. The albino rats weighing between 150 - 180 g were starved for 18 h prior to the experiment. The animals were weighed, marked for identification and divided into 17 groups. Each group contains 6 animals. Edema was induced in the left hind paw of all rats by subcutaneous injection of 0.1 mL of 1% (W/V) carrageenan in distilled water into their footpads. The 1st group was kept as control and was given the respective volume of the solvent (0.5 mL distilled water). The 2nd to 16th groups were orally administered aqueous suspension of the synthesized compounds in dose of (20 mg/kg) 1 h before carrageenan injection. The last group (standard) was administered diclofenc sodium in a dose of 20 mg/kg, orally as aqueous suspension [32] . The paw volume of each rat was measured immediately by mercury plethysmometer, before carrageenan injection and then hourly for 4 h post administration of aqueous suspension of the synthesized compounds. The edema rate and inhibition rate of each group were calculated as follows, (Edema rate (E)% = Vt − Vo/Vo, Inhibition rate (I)% = Ec − Et/Ec where Vt is the volume before carrageenan injection (mL), Vt is the volume at ten hours after carrageenan injection (mL) Ec, Et the edema rate of control and treated groups, respectively.
4.2.3. Analgesic Activity Using Hot-Plate Test
The experiment was carried out as described by Turner [33] , using hotplate apparatus, maintained at 53˚C ± 0.5˚C. The mice were divided into 17 groups of 6 animals each. The reaction time of the mice to the thermal stimulus was the time interval between placing the animal in the hot plate and when it licked its hind paw or jumped. The reaction time was measured prior to aqueous suspension of synthesized compounds and drug treatment (0 min). Group 1 was kept as normal control. The aqueous suspension of synthesized compounds was orally administered to mice of groups 2 to 16 at doses of 20 mg/kg. Mice of group 17 (reference) were orally treated with diclofenac sodium in a dose of 20 mg/kg body wt. The reaction time was again measured at 15 min. and repeated at, 30, 60 and 90 min after treatment. To avoid tissue damage to the mice paws, cut-off time for the response to the thermal stimulus was set at 60 sec. The reaction time was calculated for each synthesized compounds and drug-treated group.
4.2.4. Analgesic Activity (Acetic Acid Induced Writhing Response Model)
The compounds were selected for investigating their analgesic activity in acetic acid induced writhing response in Swiss albino mice, following the method of Collier et al. [34] . One hundred and two mice were divided into 17 groups (six in each group) starved for 16 h pretreated as follows, the 1st group which served as control positive was orally received distilled water in appropriate volumes. The 2nd to 16th groups were received the aqueous suspension of synthesized compounds orally at dose (20 mg/kg). The last group was orally received diclofenac sodium in a dose of 20 mg/kg. After 30 min, each mouse was administrated 0.7% of an aqueous solution of acetic acid (10 mL/kg) and the mice were then placed in transparent boxes for observation. The number of writhes was counted for 20 min after acetic acid injection. The number of writhes in each treated group was compared to that of a control group. The number of writhing was recorded and the percentage protection was calculated using the following ratio (%) protection = (control mean-treated mean/control mean) × 100.
NOTES
*Corresponding author.