1. Introduction
Sulfur dioxide is one of the most important pollutants of the atmosphere. It is clear that about 90% of the generated SO2 comes from industrial plants. It has many negative effects on human health. Furthermore, this atmospheric pollutant generally acidifies rainwater, which involves the deterioration of forests, the erosion of materials and the acidification of soils and fresh waters. Hence, the removal of SO2 from flue gases has received considerable attention and assumes significant importance over years due to its various deleterious effects to all forms of life [1] . Among the various physic-chemical wet and dry methods, wet scrubbing is considered to be the simplest and the most widely used process to prevent SO2 emissions and minimize its level in the atmosphere. Nevertheless, the use of the scrubbing process does not meet the legislation requirements and remains a considerable problem [2] . Moreover, the main problems in the application of the wet cleaning are the non-de- sirable byproducts like waste water, which must be disposed of or recycled. Therefore, the objective of the present work is to introduce a new method for the treatment of sulfur dioxide released from effluents of the sulfuric acid production units and the production of a mass quantity of hydrogen. This latter is a non-toxic gas whose burning behavior is highly energy producing. In fact, the production of hydrogen is interesting in a sense that it does not only generate heat by direct combustion but also generate electricity in fuel cells, with residue water only. Fuel cells are the chosen field for the use of hydrogen and can be applied in portable electronic devices, transportation or buildings [3] - [5] . Hydrogen is produced from fossil fuels (oil, gas, coal) and from water by electrolysis or thermo-chemical dissociation. Currently hydrocarbons contribute with over 90% of the traditional hydrogen production with the predominance of natural gas, but these techniques result in the release of CO2. However, because of the growing concern of the global environmental issues and the potential problems of energy resources depletion, it is highly desirable to produce hydrogen. In addition, electrolysis allows the mass production of hydrogen without greenhouse gases emissions.
This work presents a new process with two main goals. It treats SO2 and produces hydrogen. Finally, this novel method is very promising in energy and ecological terms given that hydrogen is the main factor that will promote a new air clean development as well as renewable and sustainable energy.
2. Hydrogen Production Cycles
The hydrogen production cycles performed by dissociation can be categorized as [6] - [13] :
・ Thermo-chemical hydrogen production: can be involved by direct dissociation of water or by a set of reactions at moderate temperature levels;
・ Electrochemical: can be realized by conventional or high temperature electrolysis;
・ Thermo-electrochemical: refers to the association of a low-potential electrolysis and one or more thermo-chemical reactions.
The Westinghouse hybrid cycle (Thermo-electrochemical cycle) was first proposed at Westinghouse Electric Corp. This method can be considered as a variant of sulfur-iodine cycle.
These processes consume 791 kJ of thermal energy per H2 mol produced. Of this quantity 533 kJ is consumed by the decomposition step which reaches about 67% of the total energy consumed by the process [3] [9] . This is shown in Figure 1.
The hybrid sulfur process for the production of hydrogen using a high-temperature cycle is better than the water electrolysis.
3. The Novel Process
3.1. Industrial Sulfuric Acid Production Process
The sulfuric acid industrial production (H2SO4) was realized in three steps [14] :
- SO2 production by combustion described in (R.1):
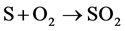 (R.1)
(R.1)
- Manufacturing SO3 by a double absorption given in (R.2):
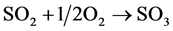 (R.2)
(R.2)
- Manufacturing H2SO4 as in (R.3):
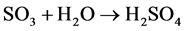 (R.3)
(R.3)
But during the production of sulfuric acid there is always an emission of SO2 into the atmosphere. Sulfur dioxide is an atmospheric pollutant which needs to be treated.
![]()
Figure 1. The Westinghouse hybrid cycle.
3.2. Using the Regenerated Sulfur Dioxide
The aim of this investigation was to integrate the electrolysis phenomenon after manufacturing the sulfuric acid in order to produce hydrogen in a massive quantity, to recover an amount of the produced sulfuric acid and also to eliminate the SO2 emission discharged from the chimney into the atmosphere.
In this novel method the reactions of sulfuric acid decomposition performed at high temperature were eliminated since we used the SO2 previously removed from the sulfuric acid production unit. Furthermore, reaction so f the sulfur iodine cycle were replaced by the sulfur dioxide electrolysis as presented by reaction (R.4).
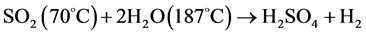 (R.4)
(R.4)
In the electrolysis step, the sulfur dioxide was dissolved in an aqueous solution anodically oxidized; the hydrogen was produced at the cathode according to reactions (R.5) and (R.6):
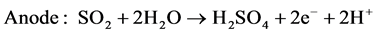 (R.5)
(R.5)
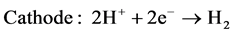 (R.6)
(R.6)
Figure 2 shows a schematic illustration of the new process.
4. Dimensioning of the New Process
4.1. Calculating the Amount of Regenerated Sulfur Dioxide
To calculate the amount of sulfur dioxide regenerated by the sulfuric acid production unit we had to achieve the molar balance of the entire process.
Data of the sulfuric acid process, such as the flow rates, temperatures and concentrations, etc. were taken from the industrial company (Tunisian Chemical Group).
For calculations, we assumed that [14] :
・ The performance of the double absorption column method was 100%;
・ The yield of the catalytic converter was of about 99.6%;
・ The sulfur dioxide mole fraction at the furnace outlet was 10.85%;
・ An output of the order of1.500 t/d of the sulfuric acid was considered as a computing basis.
Figure 3 shows a block diagram of the double absorption process. Table 1 and Table 2 give the molar flow
![]()
Figure 2. A schematic representation of the part added to the process of the novel.
![]()
Figure 3. Schematic diagramof sulfuric acid production process.
![]()
Table 1. Molar flow entering and exiting the furnace.
inlet and outlet in the process.
 (1)
(1)
x is the excess air calculate experimentally.
The molar flow rate of total gas outlet the furnace:
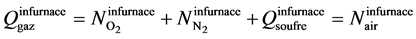 (2)
(2)
![]()
Table 2. Molar flow exiting of beds and absorbers.
R1, R2, R3 and R4 are respectively the conversion rate relative to SO2 in the bed 1, bed 2, bed 3 and bed 4.
X1 and X2 are respectively the rate of absorption relative to SO3 in the intermediate absorber and final absorber.
The molar flow rate of total gas output the bed 1:
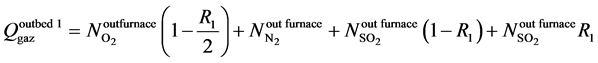 (3)
(3)
The molar flow rate of total gas output the bed 2:
 (4)
(4)
The molar flow rate of total gas output the bed 3:
![]() (5)
(5)
The molar flow rate of total gas output the intermediate absorber:
![]() (6)
(6)
The molar flow rate of total gas output the bed 4:
![]() (7)
(7)
The molar flow rate of total gas output thefinal absorber:
![]() (8)
(8)
Calculation results are presented in Table 3.
Therefore, the amount of the released SO2 was about 0.669 mol/s for the double absorption process and was about 2.683 mol/s for the simple absorption.
4.2. The Electrolysis Section
The SO2 treatment and the H2 production took place in electrolysis section. The single electrolyzer is identical for the double or simple absorption process. But the total number of electrolyzers depends on the amount of SO2.
4.2.1. Single Electrolyzer
A single electrolyzer was studied, and then the results obtained were used to study the whole electrolysis system.
![]()
Table 3. Molar fractions calculation resultsto the double absorption.
The electrolyzer used was of type PEM (Proton Exchange Membrane). Schematic of the electrolyzer system is given in Figure 4.
The following assumptions and conditions were used in the calculations:
・ The membrane used was a Nafion 212;
・ The conversion rate in the electrolyzer PEM was about 50%;
・ The temperature was T = 80˚C;
・ The pressure differential across the membrane was 600 kPa;
・ The current density was 0.5 A/cm2;
・ The surface was 10 cm × 10 cm.
According to John A. [15] - [17] , if the current density is 0.5 A/cm2, the potential is 0.71 V, the water flux production rate is 2.45 × 10−5 mol/cm2・s, and the sulfuric acid flux production rate is 0.25 × 10−5 mol/cm2・s.
Since the surface of the membrane was selected 10 × 10 = 100 cm2, then the number of moles of water flux production rate was 245 × 10−5 mol/s and the sulfuric acidflux production rate was 25 × 10−5 mol/s.
Therefore, the sulfuric acid mass fraction can be found with a simple calculation. Under these conditions the sulfuric acid produced was 35wt.%.
Maximilian [9] [18] gave the SO2 solubility in sulfuric acid as a function of acid concentration in system temperature 80˚C and the pressure is 1 bar. The solubility was found to be of 0.71 g SO2 per 100 g sulfuric acid solution. Accordingly 0.833 × 10−5 mol/s were drawn into 25 × 10−5 mol/ssulfuric acidflux production rate. Figure 5 shows a schematic of the electrolyzer model.
Since the SO2 conversion rate was 50%, then the number of moles of SO2 at the entrance of the electrolyze was the double of the amount which reacted. So the SO2 molar flux at the entrance of the electrolyzer was 50 × 10−5 mol/s. In the former 0.83 × 10−5 mol/s dissolved in sulfuric acid and the rest 24.16 × 10−5 mol/s remained outside the electrolyzer at gaseous state.
Figure 5 shows the different flows at a single electrolyzer cell and Table 4 shows a molar balance.
4.2.2. The Total Number of Electrolyzers for the Double Absorption Process
The total number of electrolyzers (![]() ) was the ratio of the total number of moles of SO2 (0.669 mol/s) per the number of moles of SO2 by a single electrolyzer (25, 84 × 10−5 mol/s). The result of the calculation was
) was the ratio of the total number of moles of SO2 (0.669 mol/s) per the number of moles of SO2 by a single electrolyzer (25, 84 × 10−5 mol/s). The result of the calculation was ![]() = 2589 electrolyzers.
= 2589 electrolyzers.
Using the Equation (9) applied to the water electrolysis and the fuel cell [19] to compute the total number of electrolyzers:
![]() (9)
(9)
![]() is the hydrogen generation rate (mol/s),
is the hydrogen generation rate (mol/s), ![]() is the electrolyzer cell’s current (A), F is the Faraday constant, 96487 (C/mol), ns is the number of electrolyzer cells connected in series, np is the number of parallel string of electrolyzer cells and
is the electrolyzer cell’s current (A), F is the Faraday constant, 96487 (C/mol), ns is the number of electrolyzer cells connected in series, np is the number of parallel string of electrolyzer cells and ![]() is the Faraday’s yield.
is the Faraday’s yield.
![]()
Figure 4. Schematic of the electrolyzer model.
![]()
Figure 5. The different flows at a single electrolyzer cell.
![]()
Table 4. A molar balance at a singleelectrolyzer cell.
The total number of single electrolyzers was:![]() .
.
This number seems very large. This is explained by the fact that the area of the membrane selected is low. But if the area is 100 cm × 100 cm the number of electrolyzer is almost 26.
Almost the same total number of single electrolyzers was found by both metods. As a result, we can conclude that this equation can be applicable in hydrogen production by sulfur dioxide electrolysis.
For a single electrolyzer, the current was 50 A and the potential was 0.71 V [15] . To connect the electrolyzers we can for example place (ns, np) = (500, 5); with ns is the number of electrolyzer in series and np is the number of branch in parallel. The total potential and the total current were determined as follows:
The total current (It) was equal to 355 V (0.71 V × 500 elec = 355 V);
And the total potential (Ut) was equal to 250 A (50 A × 5 branch = 250 A).
4.3. Sizing the Solar Panel
The characteristics of the chosen photovoltaic module (NT-175U1), which was manufactured by the SHARP Company, are summarized in Table 5.
4.3.1. Number of Branches Mounted in Parallel
A photovoltaic cell is a current generator; hence the current in the branch remains constant regardless of the number of cells connected in series. To get a well-defined quantity of current, branches must be mounted in parallel. Indeed the number of branches mounted in parallel is the ratio between the desired total current and the current of a module. In our case, the number of branches (Np) was equal to 51.
4.3.2. Number of Modules per String
The branch voltage is the sum of the modules voltages connected in series. In fact, the number of a photovoltaic cell in series (Ns) was equal to 10.
A method for optimal sizing of an electrolyzer directly connected to a PV module is the subject of further work.
In total we had to use 510 (Ns × Np = 10 × 51) Solar Panel module (NT-175U1).
5. Conclusions
This new process is able to treat 3.7 t/d of the sulfur dioxide, to recover 5.6 t/d of 35wt.% sulfuric acid and to produce 0.116 t/day of hydrogen (in the double absorption process).
A hybrid sulfur process for the production of hydrogen using a high-temperature cycle is competitive with water electrolysis.
The sulfuric acid decomposition step which produces oxygen and sulfur dioxide, is performed at a high temperature between 400˚C and 600˚C. This step is estimated to consume as much as 67% of the energy requirement for the process. So its elimination is very beneficial.
The method of using sulfur dioxide generated by the sulfuric acid production process can not only eliminate the decomposition step of the two acids but it also treats the sulfur dioxide emissions into the atmosphere. The treatment of sulfur dioxide and hydrogen production is two important aims. The produced amount of hydrogen by this method is very large.
The total number of single electrolyzers obtained when we size the electroyzer by the use of the experimental data is consistent with that obtained by the use of the equation of water electrolysis and fuel cell. So we can conclude that this equation can be applicable in a hydrogen production process by sulfur dioxide electrolysis.
A detailed economical and energetic analysis will be needed, including the cost of the electrolyzers and the solar panel. This will be the subject of a future study, which will quantify the cost of the H2 production.
An alternative optimum sizing method will be suggested to track the maximum power points (MPP) of the PV naturally in directly connected PV/PEM electrolyzer.
Acknowledgements
The authors would like to acknowledge the accountable of the research in G.C.T. because it has allowed us to do measures inside the sulfuric factory and for his encouragement about this subject.
![]()
Table 5. Solar Panel module characteristics (NT-175U1).
NOTES
*Corresponding author.