Equilibrium, Kinetic and Thermodynamics Studies of Adsorption of Aniline Blue from Aqueous Media Using Steam-Activated Carbon Prepared from Delonix regia Pod ()
1. Introduction
The impact of dyes present in water streams has being of environmental concern on the quality of such water and the aquatic animals within the water as well as its immediate surroundings. Characteristically, these dyes are persistent in nature and absorbed sunlight, which are very important for basic activities such as photosynthesis that sustain the aquatic lives in the water stream. Similarly, the presence of the dyes reduces dissolved oxygen concentration of the aquatic environment leading to increased level of COD [1] . Dyes are made of harmful organic and inorganic chemicals, which have lethal and carcinogenic effects on animals and plants in the environment [2] . The discharge of dye-contaminated wastewater, into natural streams and rivers, is mainly sourced from industries such as textile, paper, carpet, and others, which use dyes and pigments to colour their products. These dyes include natural and synthetic types. Aniline Blue has been an important dye discharging into the water stream. Ingestion of Aniline Blue results in some adverse reactions in certain organs of the human [3] ; contact with external organs like eyes results in severe irritation [4] while inhalation gives rise to difficulty in breathing [5] .
Various wastewater treatment methods including, adsorption, coagulation-flocculation, biodegradation, ion exchange, chemical oxidation, ozonation, reverse osmosis, membrane filtration and electrochemical methods, have been adopted for the removal of dye contents from various wastewater effluents [6] [7] . The suitability of these methods are currently challenged based on their cost, efficiency and environmental impact [8] ; however, adsorption treatment method has received wider application, despite these challenges [9] [10] .
Aniline Blue has been adsorbed with various agriculture wastes such as teak tree bark [11] , sunflower seed husk [12] , hazelnut shell [13] , peanut hull [14] , rice husk [15] , banana peel [16] , sugarcane bagasse [17] , wheat straw [18] , sugarcane baggase [19] , yellow passion fruit [20] , mango seed kernel [21] , and guava leaf powder [22] .
The aim of this study is to produce steam-activated carbon from local agricultural waste (Delonix regia pod) and assess the efficiency of the adsorbent produced for the removal of the Aniline Blue from aqueous solution.
2. Materials and Method
The Delonix regia pod (DRP) used for the production of natural adsorbent in this study was obtained from the Agricultural fields of Ladoke Akintola University of Technology (LAUTECH), Ogbomoso, Nigeria. The reagents used are analytical grades and were used without further purification.
2.1. Preparation of Adsorbent
The Delonix regia pods were washed extensively with distilled water to remove soil and dirt particles. They were sundried for 7 days and then crushed into smaller pieces, mechanically [23] . They were later carbonized at 300˚C for 2 h in an electric furnace and the carbonized samples were ground into granules before activation with steam in a fabricated steam activation reactor. The resultant steam activated Delonix regia carbon (SADRC) was dried inside electric oven at 105˚C and cooled at room temperature. They were ground further and sieved to 2 mm mesh size, before storage in a desiccator to avoid moisture absorption, until further use.
2.2. Preparation of Adsorbate
The adsorbate was prepared by weighing 1 g of Aniline Blue dye powder and dissolving it in small quantity of distilled water in 1000 mL capacity volumetric flask. Distilled water was then added to make up to the mark of the 1000 mL volumetric flask and then stirred rigorously for 5 min to ensure homogeneity and this makes the stock solution (1000 mg/L). Varying concentrations (50 - 200 mg/L) of the stock solution were prepared by serial dilution using Equation (1)
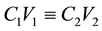 (1)
(1)
where C1 is the initial dye concentration; C2 is the final dye concentration; V1 is the initial volume of solution and V2 is the final volume of solution.
2.3. Batch Adsorption Process
Batch adsorption was conducted by weighing (0.5 - 2.0 g) of the activated carbon into sample bottles and 100 mL of various concentrations (50 - 200 mg/L) of stock solution maintained at pH 3, 4, 5, 6, 8, 9, 10 and 11 were poured into different sample bottle. Each sample bottle was shaken in a water bath shaker (Uniscope SM 101) at varied time (0 - 100 min). The solutions were removed from water bath shaker, filtered and the residue kept for further analysis such as scanning electron microscope, while the amount of dye adsorbed (qe) was quantified according to Equation (2) and the removal efficiency (RE) was determined from Equation (3).
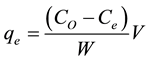 (2)
(2)
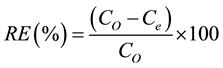 (3)
(3)
where qe (mg/g) is the quantity of Aniline Blue adsorbed per unit mass of activated carbon, CO (mg/L) is the initial Aniline Blue concentration, Ce (mg/L) is the Aniline Blue concentration after adsorption, V (mL) is the volume of the solution and W (g) is the mass of activated carbon.
3. Results and Discussion
3.1. Characterization
FTIR analysis, (Perkin-Elmer Spectrum GX, Kuala Lumpur, Malaysia) was employed to identify functional groups responsible for adsorption on the surface of the SADRC produced.
The FTIR spectra of SADRC before and after Aniline Blue adsorption onto the activated carbon developed from the Delonix regia pod (SADRC), were compared. The FTIR spectra before adsorption were in the range of 418 to 2370.81 cm−1 (Figure 1), while spectra after adsorption ranged from 426.49 and 1560 cm−1 (Figure 2), and these changes suggested that Aniline Blue adsorbed onto the SADRC. The stretching vibration band at
![]()
Figure 1. The FTIR spectra of SADRC before adsorption.
![]()
Figure 2. The FTIR spectra of SADRC after adsorption.
2169.54 cm−1 may be due to strong CN, while the stretching vibration band at around 1580 - 1650 cm−1 may be due to C=C stretching vibration. The bands around 1350 and 426.49 cm−1 are due to C-N and -SO3H group respectively and this further suggests that some functional groups may be present on the surface of the carbon due to the low temperature of carbonization (300˚C) of the adsorbent.
Plate 1(a) and Plate 1(b) show the SEM micrographs of SADRC samples before and after dye adsorption. The SADRC exhibits a caves-like, uneven and rough surface morphology. The surface of dye-loaded adsorbent, however, shows that the surface of SADRC is covered with the dye molecules.
3.2. Effect of pH on Adsorption of Aniline Blue onto SADRC
The effect pH on the removal of Aniline Blue from aqueous solution was studied by using 0.5 g dosage of the SADRC, 100 mg/L initial concentration, and 30 min. contact time. Figure 3 shows that percentage removal of Aniline Blue increased from 97.1% - 97.5% as the pH increased from pH 3 - 10, but decreased to 97.44% at pH 11. This is because pH affects the solubility of the dye, concentration of the counter ions on the functional groups of the adsorbent (SADRC) and the degree of ionization of the adsorbate during reaction [24] . When the pH of the adsorbing medium increased from pH 3 - 10, there was a corresponding increase in deprotonating of the adsorbent surface leading to a decrease in the H+ ion on the surface of the adsorbent [25] . This creates more negative charges on the adsorbent surface, thus, favouring adsorption of positively charged species onto the adsorbent (SADRC) surface [26] . The optimum removal efficiency (97.53%) ranged between pH 9 and 10.0.
3.3. Effect of Contact Time and Initial Dye Concentrations on the Adsorption of Aniline Blue onto SADRC
The adsorption of the initial concentration (100 - 200 mg/l) of Aniline Blue was investigated at various temperatures (20˚C - 50˚C) under agitation time ranging from 0 - 100 mins (Figures 4(a)-(d)). Generally, the adsorption of the dye increases as the initial concentrations of the dye increased at all the temperature. Similarly, equilibrium was attained after first 60 min for all the concentrations studied except 100 and 200 mg/l at 20˚C (Figure 4(a)) as well as 200 mg/l at 40˚C (Figure 4(c)), where the equilibrium were attained in contact time less than 60 min. The equilibrium state indicates that there is no change in the amount adsorbed, thus, given rise to constant value where the amount of dye desorbed is proportional to the amount of dye on the adsorbent infers dynamic equilibrium [27] . The time required to attain this state of equilibrium is referred to as equilibrium time and
![]()
![]() (a) (b)Plate 1. Scanning electron microscope of (a) fresh SADRC and (b) dye adsorbed SADRC.
(a) (b)Plate 1. Scanning electron microscope of (a) fresh SADRC and (b) dye adsorbed SADRC.![]()
Figure 3. Effect of pH as a function of percentage removal of AB.
the amount of Aniline Blue adsorbed at equilibrium indicates the maximum adsorption capacity of the adsorbent under the operating conditions [28] .
3.4. Effect of Adsorbent Dosage on the Adsorption of Aniline Blue onto SADRC
The adsorption of Aniline Blue on SADRC was studied at pH 10 with varying amount (0.5 - 2.0 g) of the SADRC while keeping the initial concentration of the Aniline Blue solution constant, since it has been established above that the adsorption of the dye increases as the initial concentrations of the dye increased at all the temperatures. The result (Figure 5) shows that the removal efficiency increases with increase in adsorbent dosage and this may be because increase in the adsorbent dosage increases the number of available adsorption sites and consequently increase the amount of Aniline Blue adsorbed. This further justifies that the effectiveness of an adsorption process is function of the amount of adsorbent used [29] .
3.5. Kinetic Studies
The solute uptake rate is usually described by kinetic of sorption and, consequently governs the residence time or sorption. The kinetic of Aniline Blue adsorption on Delonix regia pod was analyzed using pseudo first order and pseudo second order kinetic models. The correlation coefficient (R2) and sum of error squares (SSE%) were employed to express and validate conformity between experimental and the calculated data, respectively. Relatively high R2-values and low SSE (%) characterize the model that describes, successfully, the kinetics of Aniline Blue adsorption.
![]()
Figure 5. Percentage removal against adsorbent dosage for the adsorption of AB onto SADRC.
 (4)
(4)
where N is the number of data points.
3.5.1. The Pseudo First Order Kinetic Model
The rate constant of adsorption is determined from the pseudo first order equation given by
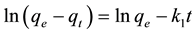 (5)
(5)
where 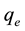 and
and ![]() are the amount of dye (mg/g) adsorbed at equilibrium and time t (min), respectively, while
are the amount of dye (mg/g) adsorbed at equilibrium and time t (min), respectively, while ![]() is the rate constant of adsorption (min−1). The values of
is the rate constant of adsorption (min−1). The values of ![]() were evaluated from the plot of
were evaluated from the plot of ![]() versus t for different initial concentrations and temperatures (Figure 6).
versus t for different initial concentrations and temperatures (Figure 6).
3.5.2. The Pseudo Second Order Kinetic Model
The pseudo second order kinetic equation based on equilibrium adsorption is expressed as:
![]() (6)
(6)
where k2 (g/mg・min) is the rate constant of pseudo second order adsorption. A plot of ![]() versus t gave a linear graph while k2 and
versus t gave a linear graph while k2 and ![]() were evaluated from the slope and intercept of the graph, respectively (Figure 7).
were evaluated from the slope and intercept of the graph, respectively (Figure 7).
3.5.3. Test of Kinetic Models
The correlation value (R2) of the pseudo first order kinetics ranged from 0.08 to 0.85 while the R2 of the pseudo second order kinetics ranged from 0.963 to 0.997 at all the temperatures and initial concentrations considered (Table 1). This suggests that the adsorption kinetics of Aniline Blue onto SADRC pod can be well represented with pseudo second order kinetic model. The suitability of the two selected kinetic models was further verified with the Sum of Error Squares (SSE%) (Equation (4)). Higher R2-values and lower SSE (%) value indicate the goodness of the fit and consequently the most suitable experimental condition for adsorption of Aniline Blue onto SADRC. The most suitable condition, in this study, occurred at 20˚C and 50 mg/l, corresponding to 0.987 (R2) and 0.077 (SSE%) under the pseudo second order kinetics model.
![]()
Table 1. Rate constants derived from pseudo first and pseudo second order adsorption kinetic model for the adsorption of AB onto SADRC at various temperatures (20˚C - 50˚C).
3.6. Isothermal Studies
3.6.1. Langmuir Adsorption Isotherm
Two Langmuir equations were applied in studying the Langmuir adsorption isotherm. The linearized form of Langmuir-1 and Langmuir-2 adsorption isotherms are expressed in Equations (7) and (8), respectively.
![]() (7)
(7)
![]() (8)
(8)
A plot of ![]() versus
versus![]() , in Equation (6), gives a straight line (Figure 8(a)) from which
, in Equation (6), gives a straight line (Figure 8(a)) from which ![]() and
and ![]() values are determined from the slope and intercept, respectively. Similarly, the plot of
values are determined from the slope and intercept, respectively. Similarly, the plot of ![]() versus , in Equation (7), gives a straight line (Figure 8(b)) from which
versus , in Equation (7), gives a straight line (Figure 8(b)) from which ![]() and b values are determined from the slope and intercept respectively. The values were reported and compared in Table 2, while the suitability of either Langmuir (Langmuir 1 and 2) was based on the correlation coefficient (R2) values. The closer the R2 values to 1, the better the fit; this indicates favourable adsorption [30] . The correlation value (R2) of the Langmuir-1 adsorption isotherms ranged from 0.596 to 0.805 while the R2 of the Langmuir-2 adsorption isotherms ranged from 0.916 to 0.979 at all the temperatures (Table 2). This suggests that the Langmuir-2 adsorption isotherms describe the adsorption of Aniline Blue onto SADRC, better than Langmuir-1 adsorption isotherms.
and b values are determined from the slope and intercept respectively. The values were reported and compared in Table 2, while the suitability of either Langmuir (Langmuir 1 and 2) was based on the correlation coefficient (R2) values. The closer the R2 values to 1, the better the fit; this indicates favourable adsorption [30] . The correlation value (R2) of the Langmuir-1 adsorption isotherms ranged from 0.596 to 0.805 while the R2 of the Langmuir-2 adsorption isotherms ranged from 0.916 to 0.979 at all the temperatures (Table 2). This suggests that the Langmuir-2 adsorption isotherms describe the adsorption of Aniline Blue onto SADRC, better than Langmuir-1 adsorption isotherms.
![]()
![]() (a) (b)
(a) (b)
Figure 8. Langmuir-1 (a) and Langmuir-2 (b) adsorption isotherm plots for the adsorption of AB onto SADRC at different temperatures.
![]()
Table 2. Langmuir isotherm constants for AB adsorption onto SADRC at various temperatures.
3.6.2. Freundlich Adsorption Isotherm
The linearized form of Freundlich Adsorption Isotherm model is often represented by Equation (9).
![]() (9)
(9)
where ![]() the amount of Aniline Blue is adsorbed at equilibrium (mg/L),
the amount of Aniline Blue is adsorbed at equilibrium (mg/L), ![]() is the equilibrium concentration of the adsorbate (mg/L), while n and
is the equilibrium concentration of the adsorbate (mg/L), while n and ![]() are constants, which incorporate the factors affecting the intensity of adsorption and adsorption capacity, respectively.
are constants, which incorporate the factors affecting the intensity of adsorption and adsorption capacity, respectively.
A plot of ![]() versus
versus ![]() gave straight line from which n and
gave straight line from which n and ![]() are evaluated as the slope and intercept of the line, respectively (Figure 9) Won et al., (2006), [31] , suggested that value of
are evaluated as the slope and intercept of the line, respectively (Figure 9) Won et al., (2006), [31] , suggested that value of ![]() less than 1 indicates favourable adsorption and formation of relatively stronger bond between the adsorbate and the adsorbent. The values of 1/n obtained in this study (Table 3) ranged between 0.309 and 0.408, which indicates that the adsorption of Aniline Blue onto SADRC at different temperatures is favourable. The highest R2 (0.955) was obtained for adsorption process conducted at 20˚C.
less than 1 indicates favourable adsorption and formation of relatively stronger bond between the adsorbate and the adsorbent. The values of 1/n obtained in this study (Table 3) ranged between 0.309 and 0.408, which indicates that the adsorption of Aniline Blue onto SADRC at different temperatures is favourable. The highest R2 (0.955) was obtained for adsorption process conducted at 20˚C.
3.7. Thermodynamic Studies
The thermodynamic of the adsorption of Aniline Blue onto SADRC at different temperatures carried out using the vant Hoff equation (Equation (10)) and the Gibbs free energy ![]() was determined from Equation (11).
was determined from Equation (11).
![]() (10)
(10)
![]() (11)
(11)
where ![]() (equilibrium constant) is equivalent to
(equilibrium constant) is equivalent to![]() , T is temperature in K, and R is the gas constant.
, T is temperature in K, and R is the gas constant.
A plot of ![]() against
against ![]() gave a straight-line graph from which
gave a straight-line graph from which ![]() and
and ![]() were evaluated as the slope and intercept, respectively (Figure 10). The positive values of
were evaluated as the slope and intercept, respectively (Figure 10). The positive values of ![]() and
and ![]() (Table 4) suggest endothermic and non-spontaneous nature of sorption process of the adsorption of Aniline Blue onto the SADRC at all temperatures investigated. On the other hand, the negative values of
(Table 4) suggest endothermic and non-spontaneous nature of sorption process of the adsorption of Aniline Blue onto the SADRC at all temperatures investigated. On the other hand, the negative values of ![]() indicate the existence of decreasing randomness of the solid/solution interface during the sorption of Aniline Blue onto the SADRC [32] .
indicate the existence of decreasing randomness of the solid/solution interface during the sorption of Aniline Blue onto the SADRC [32] .
![]()
Figure 9. Freundlich isotherm plot for the adsorption of AB onto SADRC at different temperatures.
![]()
Figure 10. Thermodynamic plot for the adsorption of AB onto SADRC pod at different initial temperature.
![]()
Table 3. Freundlich isotherm constants for AB adsorption onto SADRC at different temperatures.
![]()
Table 4. Equilibrium constants and thermodynamic parameters for the adsorption of AB onto SADRC at different initial concentration and temperature.
4. Conclusion
This present investigation vividly showed that Delonix regia pod could be effectively used as an adsorbent for the removal of Aniline Blue from aqueous solutions over a wide range of concentration and temperature. The experimental data correlated more reasonably with Langmuir than Freundlich based on the correlation regression (R2), while Langmuir exhibited the best fit among other isotherm models. The adsorption kinetic data followed the pseudo second order model. The value of![]() ,
, ![]() and ∆G result shows that the process was endothermic, non-spontaneous and thermodynamically feasible. Therefore it is recommended that SADRC derived from Delonix regia pod could be used for the treatment of textile wastewater.
and ∆G result shows that the process was endothermic, non-spontaneous and thermodynamically feasible. Therefore it is recommended that SADRC derived from Delonix regia pod could be used for the treatment of textile wastewater.
NOTES
*Corresponding author.