1. Introduction
One of the greatest manifestations of climate change is the increase in atmospheric CO2 concentration. During the last twelve years, the rate of increase of CO2 is 1.9 ppm yr−1 and is forecasted to be as high as 570 ppm by the middle and may reach 700 ppm or more by the end of this century [1] . Future climate change and associated impacts will vary from region to region around the globe. Projections suggest that the global temperature will increase by 1.8˚C - 4.0˚C, depending on the greenhouse emission scenario [1] . FAO and IPCC has estimated that cereals production in India would go down up to 125 mt. and an overall increase of 2.0˚C in temperature may cause almost 8% loss in farm level net revenue and around 5% in GDP [2] . The climate change impact on the productivity of rice in Punjab (India) has shown that keeping all other climatic variables remaining constant, temperature increases of 1˚C, 2˚C and 3˚C, would reduce the grain yield of rice by 5.4%, 7.4% and 25.1%, respectively [3] . More studies suggest a 2% to 5% decrease in yield potential of wheat and maize for a temperature rise of 0.5˚C to 1.5˚C in India [4] . Biomass and yield tend to decline with increasing temperature, as higher temperatures shorten crop duration, enhance respiration and reduce time for radiation interception [5] . Both CO2 and temperature are the key variables of global climate and may cause significant changes in crop productivity. There is rising evidence suggesting that many C3 crops, may respond positively to elevate atmospheric (CO2) in the absence of other stressful conditions [6] . but the beneficial direct impact of elevated (CO2) can be counteracted by other effects of climate change, such as elevated temperatures, higher troposphere ozone concentrations and altered patterns of precipitation [7] [8] . Studies on various plant species have suggested that climate changes will affect the development, growth and productivity of plants through alterations in their biochemical, physiological and morphogenetic processes [9] . The high yielding varieties of rice and wheat developed through modern technologies have contributed significantly in achieving good harvest [10] . Nevertheless, there is only limited information on dynamics of physiological parameters after the heading stage of rice under high air temperature and CO2 [11] [22] , especially the lack of researches on dynamics of physiological parameters after the heading stage. Therefore, there is a need to develop genotypes that are either tolerant to warming or to identify genotypes which perform better under predicted climate change scenarios. The aim of this study was to address the basic issues of to identify suitable rice and wheat genotypes and their physiological changes inside plant system under projected climate change using Open Top Chamber.
2. Material and Methods
2.1. Field Experiment
This study was conducted in the experimental farm of ICAR Research Complex for Eastern Region, Patna located at 25˚35'37"N latitude and 85˚05'E longitude and at an altitude of 51.8 m above mean sea level. The land area of open-top chambers (OTCs) had a level topography. The climate of the experimental site is semi-arid with dry hot summer and mild winters. The crop season of rice crop is from July to Oct. (kharif season). The soil at the experimental site belongs to the major group of Indo-Gangetic alluvium (Table 1).
![]()
Table 1. Soil characteristic of experimental site.
2.2. Crop Management
Four rice genotypes (R. Bhagwati, IR64, IR83376-B-B-24-2 and IR84895-B-127-CRA-5-1-1) were evaluated inside open top chambers (OTCs) at ICAR-RCER, Patna, in Kharif season 2014 with an objective to assess the impact of elevated CO2 and temperature (2˚C ˃ ambient) on morpho-physiological traits and yield. The treatment condition in each OTC was OTC1 (ambient condition), OTC2 (25% higher CO2 than ambient), OTC3 (25% higher CO2 + 2˚C ˃ ambient temperature) and OTC4 (2˚C ˃ ambient temperature). Fields (inside the OTCs) were dry ploughed and leveled but not puddled during land preparation. Twenty one days (21 days) old seedlings from wet bed nursery were transplanted at the rate of 2 seedlings per hill at a spacing of 20 cm × 15 cm in plots. In each plot a uniform plant stand was maintained and standard agronomic practices were followed for raising and maintenance of plants. Plots were fertilized at the rate of 90:60:40 kg N:P:K ha−1. Nitrogen was applied on three occasions (1/3each at sowing/transplanting as a basal, at 30 days and at 60 days after transplanting), while the P2O5 and K2O were applied as a basal application. The experimental plots were kept weed free by hand weeding. Three replications were maintained for each genotype in each open top chamber. Replications were randomized within the OTCs. The plot size for each replication was 1 m2 and about 33 plants per square meter were available. The observations were recorded on ten randomly selected plants per genotype per replication for all the traits, plant height (cm), as well as grain yield (t/ha).
2.3. CO2 Supply, Temperature Enrichment and Monitoring
All the OTCs were equipped with humidity, temperature and CO2 sensors. Each open top chamber was divided into four equal quadrants with water proof brick partitioning. In each quadrant 3 replications were maintained. Pure CO2 (99.7%, v/v CO2 and less than 10 ppm CO) was released from a commercial grade cylinder fitted with a regulator. Carbon dioxide concentration of air within the elevated CO2 chambers was maintained around the target concentration by a PC-based real-time data acquisition and control (DAC) system designed based on the principles described in [12] . The air sample from the middle of the chamber was drawn periodically into a CO2 sensor (NDIR, make Topak, USA) to monitor CO2 concentration. The set level of CO2 was maintained with the help of solenoid valves which were controlled by Program Logic Control (PLC) and Supervisory Control and Data Acquisition (SCADA) system running Winlog software (Make SELCO, Italy).A data logger recorded the mean CO2 within all chamber sat 15-min intervals. The CO2 supply was switched on and temperature maintained only during the daylight hours (i.e. from 09:00 to 17:00 h). In the OTCs with elevated temperature, reference temperature was obtained from the control OTC and air temperature was increased 2˚C above ambient chamber by infra red (IR) heating tubes, and controlled by the SCADA system. The chambers were washed regularly with a gentle stream of water to remove the dust and to maintain transparency.
2.4. Weather during Crop Season
Daily maximum and minimum temperatures, maximum and minimum relative humidity, daily rainfall were recorded from the meteorological observatory of the ICAR Research Complex, Patna. Mean daily maximum and minimum temperatures and relative humidity (RH) inside the OTC were recorded using data-logger (Figure 1).
2.5. Plant Sampling
Rice plants with uniform development process were tagged in each replication at heading stage in each plot. Tagged plants of three hills from each plot were sampled at anthesis stage, with flag leaves removed from plants for physiological parameter measurements as follows:
2.6. Relative Water Content
Leaf relative water content (RWC %) was estimated by recording the fresh weight, turgid weight of 0.5 g fresh leaf samples by keeping in water for 4 h, followed by drying in hot air oven till constant weight was achieved [13] .
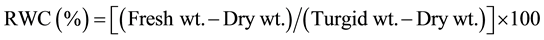
![]()
Figure 1. Average climatic condition of experimental site during Kharif season (July to October).
2.7. Membrane Stability Index
Membrane stability index (MSI %) was estimated as per [14] . Leaf material (100 mg), in two sets, was taken in test tubes containing 10 ml of double distilled water. One set was heated at 40˚C for 30 min in a metabolic water bath, and the electrical conductivity of the solution was recorded by conductivity bridge (C1). Second set was boiled at 100˚C on a boiling water bath for 10 min, and its conductivity was measured by conductivity bridge (C2). Membrane stability index was calculated as:
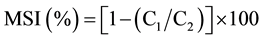
2.8. Total Chlorophyll Estimation
Estimation of chlorophyll content in plants is based on the absorption of light by chlorophyll extracts prepared by incubating the leaf tissues in DMSO (Dimethyl sulfoxide). DMSO renders plasmalemma permeable thereby, causing the leaching of the pigments [15] . Absorbance was recorded at 663 and 645 nm using DMSO as blank and was expressed as mg∙g−1 FW
Total chlorophyll = (20.2 × OD645 + 8.02 × OD663) × V/1000 × w
2.9. Net Photosynthetic Rate
Rate of photosynthesis was measured on leaves using portable Infrared Gas Analyzer (IRGA LI-6400 Model). The rate of photosynthesis was measured by operating the IRGA in the closed mode. The photosynthetic rate was determined at anthesis stage in the upper most fully expanded leaf between 10 a .m and 11.30 a .m by providing artificial light source of light intensity 1200 µmol∙m−2∙S−1. The net photosynthetic rate was expressed as µmol∙m−2∙s−1.
2.10. Total Soluble Sugars
Total sugar was determined the by Anthrone reagent method [16] .
One ml of sugar sample was taken and to this 4 ml solution of anthrone regent was added. The mixture is heated on a boiling water bath for 8 min followed by cooling. The optical density of green to dark green colour was read at 630 nm in UV-visible spectrophotometer (model Specord Bio-200, Analytik Jena, Germany). A blank and two freshly prepared glucose standards were also included with each set of samples.
2.11. Statistical Analysis
The data were analyzed statistically using factorial complete randomized design (CRD) and CD at 5% (p = 0.05) and ANOVA were calculated. The analysis was done using Statistics 8.1 software programme.
3. Results and Discussion
The concentration of CO2 changed from morning to evening. The concentration of CO2 was higher during morning hour after that due to increase in consumption by plants as the day progresed led to decrease in concentration inside the OTCs. Moreover, during evening hour the concentration of CO2 again rises (Figure 2). The temperatures inside OTCs were different in each OTC. A summary of temperature condition inside OTCs were presented in Table 2.
3.1. Relative Water Content (RWC%)
Relative water content of rice genotypes was measured to assess the water status of the plants inside open top chambers (Figure 3). The mean RWC was increased 5% under elevated CO2 condition across the cultivars while there was decline in mean RWC (21%) under elevated temperature condition as compared to ambient condition. Genotypic differences were also observed inside OTCs under different set of CO2 and temperature condition. Rice plants IR83376-B-B-24-2 and R. Bhagwati showed enhancement in RWC by around 7% and 5% treated with elevated CO2 as compared to plant grown under ambient condition while there was decline in RWC by around 4% and 13% grown under elevated temperature as compared ambient condition. While, in IR84895- B-127-CRA-5-1-1 and IR 64 enhancement was 2% and 6%, respectively. The decline in RWC was more in IR84895-B-127-CRA-5-1-1 and IR 64 rice genotypes, 29%and 18%, respectively due to elevated temperature. RWC declined more slowly at elevated CO2 [17] . Higher CO2 levels will influence stomatal behavior beneficially by reducing water loss through transpiration, thus increasing water use efficiency [18] .
![]()
Figure 2. Dynamics of CO2 concentration and consumption inside open top chamber (OTC 1, 2) and open field at 45, 60 and 75 days after transplanting.
![]()
Table 2. Average temperature variation inside open top chambers (OTCs) and field condition (met data) at 45, 60 and 75 days after transplanting (DAT).
3.2. Membrane Stability Index (MSI%)
Membrane stability index (%) was measured to assess the stability of the cell membrane of the plants leaf inside open top chambers under elevated CO2 and temperature condition (Figure 4). The mean MSI (11%) was increased under elevated CO2 condition across the cultivars while there was decline in mean MSI (14%) under elevated temperature condition. Rice plants IR83376-B-B-24-2 and R. Bhagwati showed enhancement in MSI by around 14% and 10% treated with elevated CO2 while there was decline in MSI by around 11% and 12% with elevated temperature as compared ambient condition. While, in IR84895-B-127-CRA-5-1-1 and IR 64 the increment in MSI due to elevated CO2 was 7% and 12%, respectively. The injury of cell membrane structure and function due to elevated temperature was also reported [19] -[21] .
3.3. Chlorophyll Content (mg/g DW)
The mean chlorophyll content was increased under elevated CO2 across the genotypes while there was decline in mean chlorophyll content under elevated temperature condition (Figure 5). Plants of IR83376-B-B-24-2 and R.
![]()
Figure 3. Relative water content (RWC %) of rice genotypes grown under different climatic condition inside open top chambers.
![]()
Figure 4. Membrane stability index (MSI %) of rice genotypes grown under different climatic conditions inside open top chambers.
Bhagwati grown under elevated CO2 conditions showed enhancement in chlorophyll content by around 24% and 23% while there was decline in chlorophyll content by around 16% and 15% when grown under elevated temperature. While, in IR84896-B-127-CRA-5-1-1 and IR 64 it was 6% and 12%, respectively. However under elevated temperature condition the decline in chlorophyll content in rice genotypes IR84895-B-127-CRA-5-1-1 and IR 64 was 27% and 18%, respectively. [22] reported that high air temperature during heading stage negatively influenced SPAD value (relative content of chlorophyll) in rice flag leaves, significant reduction occurring with the continuous increment of air temperature.
3.4. Photosynthetic Rate (µmols∙m−2∙s−1)
Study revealed that photosynthetic rate of rice genotypes under elevated CO2 treatment was significantly (p < 0.05) greater than the ambient condition (Figure 6). However, the maximum photosynthetic rate achieved by rice genotype IR83376-B-B-24-2 with elevated CO2 (i.e. 28.3). Photosynthetic rate under ambient CO2 was (20.1) significantly (p < 0.05) lower than that of elevated CO2 (26.4) treatment. Elevated temperatures have negative effect on plant photosynthetic rate. The mean photosynthetic rate was reduced (21%) under elevated temperature
![]()
Figure 5. Chlorophyll content of rice genotypes grown under different climatic conditions inside open top chambers.
![]()
Figure 6. Photosynthetic rate of rice genotypes grown under different climatic conditions inside open top chambers.
across the genotypes, however rice genotypes IR83376-B-B-24-2 was least affected due to elevated temperature. Elevated temperature have pronounced negative effect on the genotypes IR84895-B-127-CRA-5-1-1 and showed maximum decline (32%) in photosynthetic rate as compared to ambient condition. [23] [24] also reported that elevated CO2 concentrations stimulate photosynthesis, leading to increased plant productivity and modified water and nutrient cycles. Elevated CO2 concentrations may enhance potential net photosynthesis of C3 plants because ribulose-1, 5-bisphophate carboxylase/oxygenase (rubisco), an enzyme involved in both CO2 fixation and photorespiration [25] . Thus, an increase in ambient CO2 raises the leaf internal CO2 concentration and the CO2/O2 ratio at the rubisco site, favoring carboxylation over oxygenation in ribulose-1, 5-bisphosphate (RuBP). In general, approximately 60% of assimilates demanded by rice grain-filling are derived from post-anthesis photosynthetic production produced by flag leaves, which prominently contributes to grain filling. Therefore, the photosynthetic ability of flag leaves is crucial for the determination of grain yield. [11] found that the impaired net photosynthetic rate by high temperature was mainly attributed to the reduction of chlorophyll content as well as activities of activating enzyme (RuBisCO) and carboxylase (RuBP) involved in photosynthesis in flag leaves.
3.5. Total Soluble Sugar Content (mg/g DW)
Total soluble sugar content of the elevated CO2 treatment was significantly (p < 0.05) greater than the control OTC at all times (Figure 7). The mean total soluble sugar content was increased under elevated CO2 across the genotypes. The maximum total soluble sugar content achieved by rice genotype IR83376-B-B-24-2 with elevated CO2 (i.e. 4.62). Elevated temperatures have negative effect on total soluble sugar content. Rice genotypes IR83376-B-B-24-2 was least affected due to elevated temperature. Elevated temperature have pronounced negative effect on the genotypes IR84895-B-127-CRA-5-1-1 and showed maximum decline in total soluble sugar content as compared to ambient condition. Heat triggered a significant decrease in sugar contents of flag leaves, implying that photosynthetic mechanism was severely impaired along with the reduction of photosynthesis under high temperature, which was in agreement with the reports by [11] .
3.6. Yield and Yield Attributes
Results revealed that yield and yield attributes traits of all the genotypes of rice showed positive response with elevated CO2 and negative with elevated temperature (Table 3). The grain yield increased with elevated CO2 but declined with elevated temperature across the genotypes. Rice genotype IR83376-B-B-24-2 (4.81 t∙ha−1) followed by Rajendra Bhagwati (4.52 t∙ha−1) produced higher yield in 25% higher CO2 concentration (500 ppm) than other two varieties. Higher reduction in yield was observed in genotype IR84895-B-127-CRA-5-1-1 under
![]()
Figure 7. Total soluble sugar (TSS) of rice genotypes grown under different climatic conditions inside open top chambers.
![]()
Table 3. Yield contributing traits and yield of rice inside open top chambers (OTCs).
O―OTC; V―Variety; OXV―Interaction.
elevated temperature. [26] also reported that increasing CO2 concentration in the atmosphere could lead to higher crop yields. [27] found that grain yield of rice was declined by 10% for each 1˚C increase in the growing season minimum temperature above 32˚C. [28] analyzed the impacts of elevated CO2 and temperature on irrigated rice yield in eastern India by ORYZAI and info crop-rice models, and the result shows that increased CO2 concentration can increase the rice yield, which is concerned with the sterility of rice spikelet’s at higher temperature, the sowing time and the selection of genotypes.
3.7. Conclusions and Implications
Improved physiological traits (RWC, membrane stability, chlorophyll content, photosynthetic rate and TSS) may benefit rice genotypes under elevated CO2 and temperature conditions. By virtue of greater membrane stability and photosynthetic rate, IR83376-B-B-24-2 and Rajendra Bhagwati may be assumed as tolerant among the studied rice genotypes for growing in changing climatic conditions. Since rice is a staple food crop, long term studies may provide better understanding on the efficiency of such genotypes which are increasingly associated with food security.
NOTES
*Corresponding author.