Antibacterial Zinc Oxide Nanoparticle Coating of Polyester Fabrics ()
1. Introduction
Omnipresent colonization of material surfaces, especially by bacteria, is an undesired occurrence in the technical sector as well as in everyday life and can be life-threatening in the clinical area. In the case of used clothing and other fabrics, bacterial colonization causes unpleasant odors or infections. In recent years, mainly technical textiles are equipped with antibacterial properties, but nowadays the growing awareness of health and hygiene increases the demand for antibacterial materials in a wide range of applications including medicine, medical care, protective clothing, and household as well as sport and leisure time. The use of several substances for reducing bacterial colonization on textile surfaces have been reported including positively charged molecules [1] - [4] , silver [4] - [7] , copper [8] , triazin derivatives [9] [10] , chitosan [3] [11] [12] , or triclosan [3] . Because of existing disadvantages like toxicity [13] , high costs or issues of bacterial resistance of the mentioned examples [14] - [16] , we decide to apply zinc oxide (ZnO) as antimicrobial agent [17] - [19] . In several studies the antimicrobial effect of zinc oxide coatings against different bacteria can be already verified [20] - [23] .
For the development of antibacterial textiles for applications on a large scale, e.g. in medicine and for wearing apparel, it is necessary to find the optimum of ZnO concentration to effectively combat bacteria while preventing cytotoxic effects. Therefore, the main focus of our study is the investigation of the correlation between the ZnO content, the antibacterial activity and the cytocompatibility of ZnO coated textiles. Furthermore, our research addresses the improvement of the coated polyester regarding the optical appearance and especially of the permanence of the antibacterial effect. For that reason the CCVD process is applied to enhance the adhesive strength between the coating and the textile surface. The antibacterial activity of ZnO nanoparticle coated textiles has been proven using the nosocomial pathogens Staphylococcus aureus and Klebsiella pneumoniae.
2. Materials and Methods
2.1. Materials
Powder of ZnO nanoparticles was obtained from ABCR (Karlsruhe, Germany, article no. AB 255364) and IBUtec advanced materials AG (
Weimar
, Germany). In addition, ZnO nanoparticle dispersion in water (40% (w/w)) was obtained from IBUtec.
Impranil DLU (aliphatic polycarbonate ester polyether polyurethane dispersion in water, 60% solid content), Baybond PU 406 (non-ionic polyurethane dispersion in water, 34.5% solid content) and Desmodur DN (aliphatic poly-isocyanate) were provided by Bayer Material Science AG (Leverkusen, Germany).
3T3 mouse embryonic fibroblasts were purchased from LGC Standards GmbH (
Wesel
, Germany).
Staphylococcus aureus ATCC 6538 and Klebsiella pneumoniae ATCC 4352 were obtained from the Leibniz Institute DSMZ-German Collection of Microorganisms and Cell Cultures (
Braunschweig
, Germany). For cultivation of bacteria, special peptone and “lab-lemco” powder for preparation of Caso bouillon and bacteriological agar were purchased from Oxoid Deutschland GmbH (Wesel, Germany). Columbia agar plates with 5% sheep blood were acquired from bioMérieux Deutschland GmbH (Nürtingen, Germany). Physiological NaCl solution (0.9%) was purchased from Fresenius Kabi Deutschland AG (Bad Homburg, Germany). Tween 20 was obtained from Carl Roth GmbH (
Karlsruhe
, Germany).
Polyester knitted fabrics were provided by Thorey Gera Textilveredelung GmbH (
Gera
, Germany).
2.2. Coating Process
Activation of the polyester was performed using the CCVD technique with a custom-made winding construction with two of four burners and a limited coating width of 30 cm. For the process, an air flow of 600 L/min was used. The air/gas ratio was adjusted to 18.8. The precursor (30% hexamethyldisiloxane (HMDSO) in isopropanol) was added at a flow rate of 5.5 mL/min. The speed of sweep was 50 m/min and the temperature of the guide rollers was kept at 50˚C.
For preparation of the coating suspension, the ZnO powder (ABCR) or the 40% (w/w) ZnO suspension (IBUtec) were diluted with water to obtain the desired concentration of 0.25% to 1% of ZnO. ABCR nanoparticles were used as received without disaggregating. To the suspension 2% to 5% (w/w) of a binder (Baybond PU and Impranil DLU, respectively) was added.
For laboratory scale a culture dish was used for dip coating of the textiles. After storing the textiles for 5 min in the coating solution, they were removed and heated to 80˚C for 15 min and then heated to 150˚C for 30 min. During the coating procedure stirring at 750 rpm was necessary to avoid sedimentation of the nanoparticles.
The antibacterial coating in industrial scale was realized by a foulard machine (model HVF, Mathis AG, Oberhasli, Switzerland). Stirring was not possible during the coating procedure. The polyester was run with 100 cm/min through the ZnO suspension and squeezed at 0.3 MPa. Afterwards it was dried for 2 min at 100˚C and the crosslinking reaction was performed by heating in a second step for 1.5 min at 150˚C (Labdryer, Mathis AG, Oberhasli, Switzerland).
2.3. Characterization
Scanning electron microscopy (Supra 55V, Carl Zeiss SMT, Oberkochen, Germany) was used to investigate the size of the nanoparticles and the surface of coated polyester fabrics. Qualitative SEM-EDX analysis was carried out using the Quantax system with a Si (Li) detector (Röntec, Berlin, Germany). The samples were vapor coated with carbon and activated by an electron beam of 15 keV.
The zinc content of coated samples was determined by complexometric titration with a 0.1 M aqueous solution of ethylenediaminetetraacetic acid disodium salt (Aldrich, Steinheim, Germany). The zinc oxide on sample surface was dissolved by the use of 18% hydrochloric acid before analysis.
2.4. Washing Process
To evaluate the quality of antimicrobially finished fabrics, the permanence against mechanical and chemical st- ress was studied applying washing and drying cycles. The minimum number of cycles was defined correspond- ing to the designated purpose. The investigation was performed using an aqueous detergent solution as well as subsequent rinsing and drying steps in a modified wash test according to DIN EN ISO 105-C10, method A.
The washing tester “Linitest” was used for determining the wash durability of the fabrics. Two specimens with a size of 10 cm × 4 cm were treated in a steel container for 30 min at 40˚C using a liquor ratio (specimen: liquor) of 50 g/L. The used washing liquor contained 5 g/L of a detergent.
The samples were transferred to a container with 2 L of distilled water and rinsed by careful moving. The samples were then rinsed under running cold water and dried in the air.
2.5. Antibacterial Activity
Testing for antibacterial activity was carried out in accordance to the Japanese Industrial Standard (JIS L 1902:2002, “Testing method for antibacterial activity of textiles”) as reported previously [24] . In brief, S. aureus or K. pneumoniae were cultivated in caso-bouillon at 37˚C for 18 h under aerobic conditions. For experiments, bacteria cultures were diluted with Caso bouillon to an inoculum concentration of 5 × 105 cfu/mL. 400 mg samples of the test material were inoculated with 200 µL test microbe inoculum and incubated at 37˚C for 24 h under aerobic conditions. Untreated polyester was used as negative control. For determination of the germ number after 24 h, samples were extracted in 0.9% NaCl solution supplemented with 0.2% tween 20. Serial dilutions were plated onto Columbia agar plates and incubated for 24 h at 37˚C. Afterward, colonies were calculated, total cfu (colony forming units) estimated, and growth reduction assessed according to Equation (1). According to JIS L 1902, a logarithmic microbial growth reduction of less than 0.5 denotes no antibacterial activity. Values from 0.5 to 1 are estimated as a slight, values greater than 1 and less or equal to 3 as a significant, and a log reduction greater than 3 as a strong antibacterial activity.
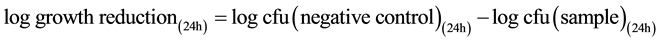 (1)
(1)
Equation (1) is the calculation of logarithmic microbial growth reduction.
2.6. Cytocompatibility
To assess cytocompatibility, the coated textiles and an uncoated sample as control were treated with 70% ethanol, washed with phosphate buffered saline (PBS) and seeded with 3T3 mouse fibroblast cells at a cell density of about 25,000 cells/cm2. The cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) containing 10% fetal bovine serum (FBS), 50 U/mL penicillin and 50 µg/mL streptomycin at 37˚C under a 5% CO2 atmosphere. The medium was renewed on day 2. After 1 and 4 days the medium was changed against fluorescein diacetate (FDA) and GelRed®. An Axiotech microscope (Zeiss, Jena, Germany) and a halogen lamp were used to monitor green and red fluorescence. Photomicrographs were recorded using a CCD microscope imaging system MP 5000 (Intas, Goettingen, Germany). Imaging was supported by Image-Pro Plus 5.1 software (Media Cybernetics, Silver Spring, USA). The total number of cells and the percentage of living cells were calculated after counting green fluorescent living cells and red fluorescent nuclei of dead cells.
3. Results and Discussion
3.1. Nanoparticles
The size of ZnO nanoparticles influences color, release of Zn ions, and particle distribution as well as roughness of the coated surface. Therefore, ZnO nanoparticles which are differ in particle size should be used to achieve optimal properties of an antibacterial coating. SEM-images of the applied nanoparticles of ABCR as well as IBUtec are shown in Figure 1.
It was found that the particles of the two suppliers vary distinctly in particle size. The mainly hexagonal prismatic ZnO NPs obtained from ABCR show a white color and a particle size in the range of 50 - 300 nm with scattered larger particles and aggregates. The ZnO particles obtained from IBUtec have a spherical shape with an average particle size of 10 nm. Due to the small size these particles exhibit a brownish color.
3.2. Coating Process
For laboratory scale, polyester samples of 4 cm × 10 cm were dip coated in a culture dish using 100 mL of an aqueous ZnO suspension (0.3% - 2.0% (w/w)) containing Impranil DLU (2% (w/w)) as binder. The binder serves as a matrix for incorporating the ZnO nanoparticles improving the adhesion of ZnO to the hydrophobic textile surface. Complexometric analyses were performed at coatings with a concentration of 1.4% (w/w) ZnO. An average amount of 0.23 mg ZnO/cm2 was found on these samples measured. Neither the particles used (ABCR or IBUtec) nor the activation with CCVD had an influence on the ZnO content of coated samples.
For coating procedures on industrial scale with a foulard machine a ZnO containing suspension (0.04% - 0.65% (w/w)) was used. Figure 2 shows graphically the determined amounts of ZnO of coated polyester samples by complexometric titration.
No linear correlation was found between applied amount of ZnO powder used from ABCR and measured amount of ZnO of coated textiles (Figure 2(a)). The coating solution with 0.32% ZnO content resulted in higher ZnO depositions on textile with CCVD pretreatment than the solution with 0.65% ZnO. This is most likely due to the deposition of the powder at the vessel bottom where it is not available during the coating procedure. This problem was avoided using the finely dispersed aqueous solution of ZnO nanoparticles from IBUtec (Figure 2(b)). Here, the measured amount of coating solution containing 0.5% of ZnO was found to be higher than the amount by using a 0.25% ZnO solution. With CCVD an even distinctly higher quantity of ZnO could be achieved because of the higher absorption properties of the hydrophilic nano layer on the surface. However, due to the incompatibility of the ZnO nanoparticle suspension with the binder Impranil, the system had to be changed to use Baybond PU for coating. Additional use of the hardener Desmodur DN leads to further increase of the ZnO content on the textiles.
![]()
![]()
Figure 1. SEM images of ZnO nanoparticles obtained from ABCR (a) and IBUtec (b).
The SEM images of laboratory coated textiles reveal the considerably higher adhesion of the ZnO/Impranil system in the case of ABCR particles (Figure 3(a)) compared to the IBUtec particles (Figure 3(b)). Nevertheless, the qualitative EDX spectra (Figure 3(c), Figure 3(d)) show the presence of Zn in both coating systems. But in the case of ABCR ZnO particles palpable white deposits on the textiles could be observed (Figure 3(e)). However the appearance of the coating clearly favors IBUtec particles despite the slight brownish color (Figure 3(f)) whereby spotting can be avoided by uniform application at industrial conditions with the foulard machine.
3.3. Antibacterial Activity
Figure 4 shows the antibacterial activity of laboratory coated polyester samples against Staphylococcus aureus and Klebsiella pneumoniae in vitro. Coating with ABCR ZnO particles and Impranil DLU as binder conveyed a strong antibacterial activity against S. aureus and K. pneumoniae up to 10 washing cycles (with and without CCVD pretreatment). In contrast, coating with IBUtec ZnO particles (with and without CCVD pretreatment) was less permanent and accomplished only a significant antibacterial effect against S. aureus and a slight antibacterial influence on K. pneumoniae after 10 washing cycles. After 50 washing cycles, textiles coated with ZnO nanoparticles of ABCR still obtained significant to strong results whereas coatings with ZnO particles of IBUtec showed a distinctly diminished antibacterial efficacy, though other studies showed an increasing activity with decreasing the particle size [22] [25] . We propose that the differences observed in the antibacterial activity of the different particles after several numbers of washing cycles are most likely due to a higher durability of ABCR ZnO particles on the polyester samples. Certainly the result based on the larger surface area of the IBUtec nanoparticles. These smaller particles will be dissolved faster during washing process.
Figure 5 shows the test results for the antimicrobial effect of the coated and washed polyester samples with the system ZnO (IBUtec, aqueous dispersion) and Baybond PU against Staphylococcus aureus and Klebsiella pneumoniae on industrial scale. Because of sedimentation of ABCR particles the IBUtec dispersion was used only for further studies. Simultaneously, the influences of the CCVD pretreatment and an additionally cross linking hardener (Desmodur DN) were investigated. No additional beneficial effect of the cross linking hardener on the washing stability of the coating could be observed. Pretreatment of the polyester samples with CCVD also showed no improvement of the permanence of the antibacterial activity. However, since pretreated textiles are more hydrophilic they can absorb more solution and the resulting amount of zinc oxide is higher than on untreated textiles. Figure 5 also shows that the washing stability by using Baybond PU is not as good as using Impranil DLU. Mainly slight antimicrobial effects could be observed after 10 washing cycles thus the influence of more than 10 washing cycles to the antimicrobial activity was not determined.
3.4. Cytocompatibility
The cytocompatibility of the coated polyester samples was studied by seeding 3T3 fibroblast cells on the surface of the textiles and then estimating cell adhesion and viability using a FDA/GelRed® live/dead assay.
![]()
![]() (a) (b)
(a) (b)
Figure 2. Measured amount of ZnO on industrial coated textiles using ZnO powder from ABCR and the binder Impranil DLU (a) as well as ZnO dispersion from IBUtec and Binder Baybond PU (b).
Results shown here are exemplarily for the concentrations of 0%, 0.25% and 0.5% ZnO (Figure 6). After an incubation period of 1 and 4 day, respectively, a large number of viable cells and only few dead ones could be detected on the control without coating. As can be seen on Figure 6(c), e that there are only a few viable cells after 1 day seeding on coated textile samples. At a concentration of 0.5% ZnO in the coating solution (amount of about 50 µg ZnO/cm2 textile) there is a strong cytotoxicity after incubation of 1 day with 45% of dead cells and
![]()
![]() (a) (b)
(a) (b)
Figure 4. Antibacterial activity (log growth reduction) of laboratory coated and washed polyester textiles with ZnO and Impranil DLU, comparison of ABCR and IBUtec-particles (n. d. = not determined).
![]()
![]() (a) (b)
(a) (b)
Figure 5. Antibacterial activity (log growth reduction) of industrial coated and washed polyester textiles with aqueous ZnO dispersion of IBUtec, Baybond PU and cross linking hardener Desmodur DN.
4 days with 23% of dead cells. The rising number of viable cells causes the decreasing number of dead cells. By changing the concentration of ZnO to 0.25% (amount of about 20 µg ZnO/cm2 textile) we found cytotoxic effects only after 1 day of incubation with an amount of 22% of dead cells. After 4 days of seeding with 3T3 fibroblast cells we found no cytotoxic effects of the coating with 3% of dead cells (Figure 6(f)). Nevertheless the total number of viable cells is 65% in comparison with the uncoated textile sample. Investigations of Punnoose et al. revealed cytotoxicity of ZnO to prokaryotic and eukaryotic cells in higher concentrations [26] . It could be shown that the lethal concentration for zebra fish embryos is in a range of 50 - 100 mg/L ZnO nanoparticles and a lower concentration (1 - 25 mg/L) was accompanied with a retarded hatching of the embryos [27] .
4. Conclusion
The antibacterial efficacy and washing stability of coated polyester samples depends on the composition of the coating solution. Particles of ZnO in the range of 50 - 300 nm demonstrate better results with regard to antibacterial efficacy after different washing cycles whereas nanoparticles with a size of 10 nm show improved optical appearance. Using the binder Impranil DLU for coating, slightly enhanced activity against Staphylococcus aureus and Klebsiella pneumoniae is obtained compared to the binder Baybond PU. Coating on an industrial scale with a foulard machine requires a finely dispersed ZnO solution for coating to avoid sedimentation of the nanoparticles. Because of the incompatibility of the zinc oxide dispersion with Impranil DLU the binder Baybond PU represents a favorable alternative for the developed coating system. Pretreatment of the textiles with CCVD-technique to generate a hydrophilic nano layer of hydroxyl terminated silicon network on the textile sur- face has no influence on the antibacterial efficacy and washing stability of the coating. But using CCVD the applied amount of ZnO can be increased on a foulard machine emphasizing the use of CCVD for industrial scale.
A ZnO concentration up to 20 µg ZnO/cm2 is found to exhibit suitable antibacterial effect and requires cytocompatibility. Further studies in order to enhance washing stability of the antibacterial coated textiles have do be done.
Funding
This work was supported by the Federal Ministry of Education and Research (grant no. 03WKBR7G).
Acknowledgements
We thank Dr. M. Schweder for SEM and EDX measurements and U. Gitter for the CCVD-treatment.
NOTES
*Corresponding author.